One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
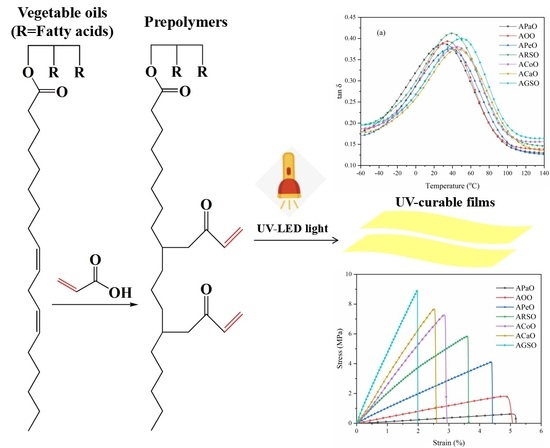
2.2. Synthesis of Vegetable Oil-Based Acrylate Prepolymers
2.3. Preparation of UV-Curable Films
2.4. Characterization
3. Results and Discussion
3.1. Structure Analysis of Vegetable Oil-Based Acrylate Prepolymers
3.2. Dynamic Mechanical Analysis (DMA)
3.3. Thermal Stability
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghaemy, M.; Bekhradnia, S. Thermal and photocuring of an acrylate-based coating resin reinforced with nanosilica particles. J. Coat. Technol. Res. 2012, 9, 569–578. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Lai, G. Fabrication of UV-curable solvent-free epoxy modified silicone resin coating with high transparency and low volume shrinkage. Prog. Org. Coat. 2019, 129, 96–100. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, G.; Hu, H. UV-curable antismudge coatings. ACS Appl. Mater. Interfaces 2017, 9, 25623–25630. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Fan, J.; Li, G. Recyclable thermoset shape memory polymers with high stress and energy output via facile UV-curing. J. Mater. Chem. A 2018, 6, 11479–11487. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Fairbanks, B.D.; Liang, H.; Ke, J.; Zhu, C. Fast self-healing engineered by UV-curable polyurethane contained Diels-Alder structure. Prog. Org. Coat. 2019, 131, 131–136. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zhao, Y.; Zhu, K.; Li, Y.; Tao, C.; Yuan, X. UV-curable POSS-fluorinated methacrylate diblock copolymers for icephobic coatings. Prog. Org. Coat. 2016, 93, 87–96. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, X.; Ou, Z.; Lin, G.; Yin, J.; Liu, Z.; Zhang, L.; Liu, X. UV-curable, 3D printable and biocompatible silicone elastomers. Prog. Org. Coat. 2019, 137, 105372. [Google Scholar] [CrossRef]
- Scarsella, J.B.; Zhang, N.; Hartman, T.G. Identification and migration studies of photolytic decomposition products of UV-photoinitiators in food packaging. Molecules 2019, 24, 3592. [Google Scholar] [CrossRef] [Green Version]
- Frewin, C.L.; Ecker, M.; Joshi-Imre, A.; Kamgue, J.; Waddell, J.; Danda, V.R.; Stiller, A.M.; Voit, W.E.; Pancrazio, J.J. Electrical properties of thiol-ene-based shape memory polymers intended for flexible electronics. Polymers 2019, 11, 902. [Google Scholar] [CrossRef] [Green Version]
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef] [PubMed]
- Juhl, M.; Mueller, J.P.B.; Leosson, K. Metasurfacepolarimeter on optical fiber facet by nano-transfer to UV-curable hybrid polymer. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Smitha, V.S.; Jaimy, K.B.; Shajesh, P.; Jeena, J.K.; Warrier, K.G. UV curable hydrophobic inorganic–organic hybrid coating on solar cell covers for photocatalytic self cleaning application. J. Mater. Chem. A 2013, 1, 12641–12649. [Google Scholar] [CrossRef]
- Gouzman, I.; Atar, N.; Grossman, E.; Verker, R.; Bolker, A.; Pokrass, M.; Sultan, S.; Sinwani, O.; Wagner, A.; Lück, T. 3D printing of bismaleimides: From new ink formulation to printed thermosetting polymer objects. Adv. Mater. Technol. 2019, 4, 1900368. [Google Scholar] [CrossRef]
- Cernadas, T.; Santos, M.; Gonçalves, F.; Alves, P.; Correia, T.R.; Correia, I.J.; Ferreira, P. Functionalized polyester-based materials as UV curable adhesives. Eur. Polym. J. 2019, 120, 109196. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Ragogna, P.J.; Xu, C.C. Methacrylation of kraft lignin for UV-curable coatings: Process optimization using response surface methodology. Biomass Bioenergy 2019, 120, 332–338. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Zhu, Y.; Xu, J.; Yao, Y. Preparation, characterization, and properties of novel ultraviolet-curable and flame-retardant polyurethane acrylate. Prog. Org. Coat. 2019, 129, 309–317. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile synthesis and characterization of novel multi-functional bio-based acrylate prepolymers derived from tung oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Q.; Li, X.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. High-fidelity and high-efficiency additive manufacturing using tunable pre-curing digital light processing. Addit. Manuf. 2019, 30, 100889. [Google Scholar] [CrossRef]
- Lee, T.H.; Park, Y.I.; Lee, S.-H.; Shin, J.; Noh, S.M.; Kim, J.C. A crack repair patch based on acrylatedepoxidized soybean oil. Appl. Surf. Sci. 2019, 476, 276–282. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Zhu, J.; Liu, X.; Wang, Z.; Yan, J. UV-curable coatings from multiarmedcardanol-based acrylate oligomers. ACS Sustain. Chem. Eng. 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Liang, B.; Li, R.; Zhang, C.; Yang, Z.; Yuan, T. Synthesis and characterization of a novel tri-functional bio-based methacrylate prepolymer from castor oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 135, 170–178. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Cao, Y.; Wang, Q.; Guo, C. Preparation and flame retardancy of castor oil based UV-cured flame retardant coating containing P/Si/S on wood surface. Ind. Crop. Prod. 2019, 130, 562–570. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.; Tang, J.; Wang, C.; Zhang, F.; Jia, P.; Feng, G.; Wu, Q.; Liu, C.; Hu, L.; et al. Use of cardanol-based acrylate as reactive diluent in UV-curable castor oil-based polyurethane acrylate resins. Ind. Crop. Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Chen, G.; Guan, X.; Xu, R.; Tian, J.; He, M.; Shen, W.; Yang, J. Synthesis and characterization of UV-curable castor oil-based polyfunctional polyurethane acrylate via photo-click chemistry and isocyanate polyurethane reaction. Prog. Org. Coat. 2016, 93, 11–16. [Google Scholar] [CrossRef]
- Eren, T.; Çolak, S.; Küsefoglu, S.H. Simultaneous interpenetrating polymer networks based on bromoacrylated castor oil polyurethane. J. Appl. Polym. Sci. 2006, 100, 2947–2955. [Google Scholar] [CrossRef]
- Eren, T.; Sefo, K.; Lu, S.H. Synthesis and characterization of copolymers of bromoacrylated methyl oleate. J. Appl. Polym. Sci. 2004, 94, 2475–2488. [Google Scholar] [CrossRef]
- Eren, T.; Kusefoglu, S.H. Synthesis and polymerization of the acrylamide derivatives of fatty compounds. J. Appl. Polym. Sci. 2005, 97, 2264–2272. [Google Scholar] [CrossRef]
- Walther, S.; Strehmel, B.; Strehmel, V. Functionalization of an alkyd resin with (meth)acrylate groups for photoinitiated polymerization. Prog. Org. Coat. 2018, 125, 316–324. [Google Scholar] [CrossRef]
- Zhang, P.; Xin, J.; Zhang, J. Effects of catalyst type and reaction parameters on one-step acrylation of soybean oil. ACS Sustain. Chem. Eng. 2013, 2, 181–187. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J. One-step acrylation of soybean oil (SO) for the preparation of SO-based macromonomers. Green Chem. 2013, 15, 641. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, Y.; Yang, Z.; Yuan, T.; Huang, J.; Li, P.; Liu, Y.; Zhang, S.; Yang, Z. Facile synthesis and characterization of urushiol analogues from tung oil via ultraviolet photocatalysis. Prog. Org. Coat. 2018, 120, 240–251. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Liu, R.; Liu, X.; Liu, J. Highly functional bio-based acrylates with a hard core and soft arms: From synthesis to enhancement of an acrylatedepoxidized soybean oil-based UV-curable coating. Prog. Org. Coat. 2019, 134, 342–348. [Google Scholar] [CrossRef]
- Ge, X.; Yu, L.; Liu, Z.; Liu, H.; Chen, Y.; Chen, L. Developing acrylatedepoxidized soybean oil coating for improving moisture sensitivity and permeability of starch-based film. Int. J. Biol. Macromol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.S. Camelina oil derivatives and adhesion properties. Ind. Crop. Prod. 2015, 73, 73–80. [Google Scholar] [CrossRef]
- Liang, B.; Kuang, S.; Huang, J.; Man, L.; Yang, Z.; Yuan, T. Synthesis and characterization of novel renewable tung oil-based UV-curable active monomers and bio-based copolymers. Prog. Org. Coat. 2019, 129, 116–124. [Google Scholar] [CrossRef]
- Esen, H.; Çayli, G. Epoxidation and polymerization of acrylated castor oil. Eur. J. Lipid Sci. Technol. 2016, 118, 959–966. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Dai, J.; Liu, X.; Jiang, Y.; Wang, S.; Wei, J.; Chen, J.; Zhu, J. Itaconicacid as a green alternative to acrylic acid for producing a soybean oil-based thermoset: Synthesis and properties. ACS Sustain. Chem. Eng. 2017, 5, 1228–1236. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Man, L.; Yuan, T.; Zhang, C.; Yang, Z. Biobased thiol-epoxy shape memory networks from gallic acid and vegetable oils. Eur. Polym. J. 2019, 112, 619–628. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, T.; Yang, Z.; Man, L.; Hu, Y.; Yang, Z. UV/thermal dual curing of tung oil-based polymers induced by cationic photoinitiator. Prog. Org. Coat. 2019, 126, 8–17. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.; Tajau, R.; Mahmood, M.H.; Yunus, W.M. Synthesis of radiation curable palm oil-based epoxy acrylate: NMR and FTIR spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, D.; Sun, X.S. Epoxidized and acrylatedepoxidizedcamelina oils for ultraviolet-curable wood coatings. J. Am. Oil Chem. Soc. 2018, 95, 1307–1318. [Google Scholar] [CrossRef]







| Samples | Double Bonds | Grafting Number | Grafting Rate (%) |
|---|---|---|---|
| APaO | 1.70 | 1.13 | 66.47 |
| AOO | 2.82 | 1.59 | 56.38 |
| APeO | 3.48 | 1.72 | 49.43 |
| ARSO | 3.81 | 1.85 | 48.56 |
| ACoO | 4.36 | 2.04 | 46.79 |
| ACaO | 4.47 | 2.06 | 46.09 |
| AGSO | 4.53 | 2.17 | 47.90 |
| Samples | Tg (°C) | E′25 (MPa) | E′ at Tg + 30 °C (MPa) | νe (×103 mol·m−3) |
|---|---|---|---|---|
| APaO | 30.3 | 63.6 | 16.8 | 2.0 |
| AOO | 35.5 | 103.2 | 24.4 | 2.9 |
| APeO | 38.3 | 209.2 | 53.9 | 6.3 |
| ARSO | 40.1 | 326.9 | 68.4 | 8.0 |
| ACoO | 45.0 | 397.5 | 74.7 | 8.6 |
| ACaO | 46.4 | 435.4 | 92.0 | 10.6 |
| AGSO | 50.0 | 565.8 | 117.3 | 13.3 |
| Samples | T10% (°C) | T50% (°C) | Char Yield (%) |
|---|---|---|---|
| APaO | 265.9 | 408.9 | 2.47 |
| AOO | 270.6 | 412.0 | 2.61 |
| APeO | 279.0 | 417.3 | 2.73 |
| ARSO | 287.5 | 418.2 | 3.15 |
| ACoO | 290.7 | 420.5 | 3.39 |
| ACaO | 291.7 | 423.4 | 3.54 |
| AGSO | 304.4 | 428.3 | 3.74 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| APaO | 0.62 ± 0.08 | 5.12 ± 0.61 | 13.93 ± 3.37 |
| AOO | 1.75 ± 0.16 | 4.91 ± 0.74 | 37.15 ± 8.61 |
| APeO | 4.10 ± 0.21 | 4.39 ± 0.13 | 94.07 ± 13.87 |
| ARSO | 5.81 ± 0.52 | 3.61 ± 0.67 | 169.82 ± 28.25 |
| ACoO | 7.26 ± 1.07 | 2.87 ± 0.54 | 259.72 ± 24.61 |
| ACaO | 7.67 ± 0.93 | 2.54 ± 0.29 | 301.85 ± 23.93 |
| AGSO | 8.94 ± 1.10 | 1.97 ± 0.09 | 468.07 ± 31.06 |
| Vegetable Oils | Number of Steps | Functionality | Tg (°C) | T50% (°C) | Tensile Strength (MPa) | References |
|---|---|---|---|---|---|---|
| Castor oil | 2 | 3 | 32.0–72.1 | 416.0–428.7 | 8.15–12.32 | [22] |
| Tung oil | 2 | 6 | 85.7–123.7 | 440.3–465.9 | 10.72–18.07 | [18] |
| Palm oil | 2 | 3 | 115.5–119.6 | 440.3–444.0 | 5.2–6.2 | [41] |
| Soybean and camelina oil | 2 | 2.5–3.3 | 43.8–67.7 | 415 | 8.9–17.0 | [42] |
| A range of vegetable oils | 1 | 1.13–2.17 | 30.3–50.0 | 408.9–428.3 | 0.62–8.94 | Present |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymers 2020, 12, 1165. https://doi.org/10.3390/polym12051165
Su Y, Lin H, Zhang S, Yang Z, Yuan T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymers. 2020; 12(5):1165. https://doi.org/10.3390/polym12051165
Chicago/Turabian StyleSu, Yupei, Hai Lin, Shuting Zhang, Zhuohong Yang, and Teng Yuan. 2020. "One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings" Polymers 12, no. 5: 1165. https://doi.org/10.3390/polym12051165