Hydrophobic Modification of Cashew Gum with Alkenyl Succinic Anhydride
Abstract
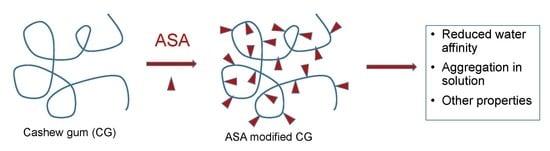
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ASA–CG
2.3. Characterization Methods
3. Results and Discussion
3.1. ASA Reactions
3.2. Spectroscopic Characterization
3.3. Thermogravimetric Analysis
3.4. Molecular Weight Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mothe, C.G.; Oliveira, N.N.; De Freitas, A.S.; Mothé, M.G. Cashew Tree Gum: A Scientific and Technological Review. Int. J. Environ. Agric. Biotechnol. 2017, 2, 681–688. [Google Scholar] [CrossRef]
- Kumar, A.; Moin, A.; Shruthi, R.; Ahmed, A.; Shivakumar, H.G. Cashew gum—A versatile hydrophilic polymer: A review. Curr. Drug Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; De Souza, F.R.L.; Bezerra, J.M.; Oliveira, C.; Novotny, D.; Soares, M.F.L.R.; Nunes, L.C.C.; Filho, E.C.D.S.; Veiga, F.; Sobrinho, J.L.S. Gums’ based delivery systems: Review on cashew gum and its derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.A.; Oliveira, M.R.F.; Furtado, R.F.; Borges, M.D.F.; Biswas, A.; Cheng, H.N.; Alves, C.R. Preparation and characterization of carboxymethyl cashew gum grafted with immobilized antibody for potential biosensor application. Carbohydr. Polym. 2019, 228. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Kim, S.; Buttrum, M.; Furtado, R.F.; Alves, C.R.; Cheng, H.N. Preparation of Hydrophobically Modified Cashew Gum Through Reaction with Alkyl Ketene Dimer. In Green Polymer Chemistry: New Products, Processes, and Applications; American Chemical Society: Washington, DC, USA, 2018; pp. 137–146. [Google Scholar]
- Candau, F.; Selb, J. Hydrophobically-modified polyacrylamides prepared by micellar polymerization1Part of this paper was presented at the conference on ‘Associating Polymer’, Fontevraud, France, November 1997.1. Adv. Colloid Interface Sci. 1999, 79, 149–172. [Google Scholar] [CrossRef]
- Associative Polymers in Aqueous Media. In ACS Symposium Series 765; Glass, J.E. (Ed.) American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Winnik, M.A.; Yekta, A. Associative polymers in aqueous solution. Curr. Opin. Colloid Interface Sci. 1997, 2, 424–436. [Google Scholar] [CrossRef]
- Landoll, L.M. Nonionic polymer surfactants. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 443–455. [Google Scholar] [CrossRef]
- Tanaka, R.; Meadows, J.; Williams, P.A.; Phillips, G.O. Interaction of hydrophobically modified hydroxyethyl cellulose with various added surfactants. Macromolecules 1992, 25, 1304–1310. [Google Scholar] [CrossRef]
- Emmons, W.D.; Stevens, T.E. Polyurethane Thickeners in Latex Compositions. U.S. Patent 4,079,028, 14 March 1978. [Google Scholar]
- Glass, J.E. Influence of Water-Soluble Polymers on Rheology of Pigmented Latex Coatings. Adv. Chem. 1986, 213, 391–416. [Google Scholar]
- Shay, G.D.; Eldridge, E.; Kail, J.E. Alkali Soluble Latex Thickeners. U.S. Patent 4,514,552, 30 April 1985. [Google Scholar]
- Shay, G.D.; Kravitz, F.K.; Brizgys, P.V.; Kersten, M.A. Production of Alkali-Soluble, Carboxyl-Functional Aqueous Emulsion Thickeners. U.S. Patent 4,801,671, 31 January 1989. [Google Scholar]
- Shay, G.D.; Kravitz, F.K.; Brizgys, P.V. Effects of Process Variables on the Emulsion and Solution Properties of Hydrophobically Modified Alkali-Swellable Emulsion Thickeners. ACS Symp. Ser. 1991, 462, 121–141. [Google Scholar]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 1. Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning polysaccharides into hydrophobic materials: A critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose 2010, 17, 1045–1065. [Google Scholar] [CrossRef]
- Qiao, L.; Gu, Q.-M.; Cheng, H. Enzyme-catalyzed synthesis of hydrophobically modified starch. Carbohydr. Polym. 2006, 66, 135–140. [Google Scholar] [CrossRef]
- Cheng, H.N.; Gu, Q.-M. Enzyme-Catalyzed Modifications of Polysaccharides and Poly(ethylene glycol). Polym. 2012, 4, 1311–1330. [Google Scholar] [CrossRef] [Green Version]
- Brander, J.; Thorn, I. Surface Application of Paper Chemicals; Blackie Academic: London, UK, 1997. [Google Scholar]
- Reynolds, W.F. The Sizing of Paper, 2nd ed.; TAPPI: Atlanta, GA, USA, 1989. [Google Scholar]
- Oppolzer, W.; Snieckus, V. Intramolecular Ene Reactions in Organic Synthesis. Angew. Chem. Int. Ed. 1978, 17, 476–486. [Google Scholar] [CrossRef]
- Nahm, S.H.; Cheng, H.N. Transition-state geometry and stereochemistry of the ene reaction between olefins and maleic anhydride. J. Org. Chem. 1986, 51, 5093–5100. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schaefer, C.; Gilbert, R.G. Sgtructure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Shah, N.; Soni, N.; Singhal, R.S. Modification of proteins and polysaccharides using dodecenyl succinic anhydride: Synthesis, properties and applications—A review. Int. J. Boil. Macromol. 2018, 107, 2224–2233. [Google Scholar] [CrossRef]
- Jeon, Y.-S.; Viswanathan, A.; Gross, R.A. Studies of starch esterification: Reactions with alkenyl succinates in aqueous slurry systems. Starch 1999, 51, 90–93. [Google Scholar] [CrossRef]
- Viswanathan, A. Effect of degree of substitution of octenyl succinate starch on the emulsion activity on different oil phases. J. Environ. Polym. Degrad. 1999, 7, 191–196. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Singhal, R.S. Studies on the optimization of preparation of succinate derivatives from corn and amaranth starches. Carbohydr. Polym. 2002, 47, 277–283. [Google Scholar] [CrossRef]
- Shogren, R. Rapid preparation of starch esters by high temperature/pressure reaction. Carbohydr. Polym. 2003, 52, 319–326. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.; Kim, S.; Willett, J. Rapid preparation of starch maleate half-esters. Carbohydr. Polym. 2006, 64, 484–487. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Synthesis of dodecenyl succinic anhydride (DDSA) corn starch. Food Res. Int. 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, L.; Tong, J.; Xie, L.; Liu, Z. Surface esterification of corn starch films: Reaction with dodecenyl succinic anhydride. Carbohydr. Polym. 2009, 78, 888–893. [Google Scholar] [CrossRef]
- Rivero, I.E.; Balsamo, V.; Müller, A.J. Microwave-assisted modification of starch for compatibilizing LLDPE/starch blends. Carbohydr. Polym. 2009, 75, 343–350. [Google Scholar] [CrossRef]
- Huang, Q.; Fu, X.; He, X.; Luo, F.; Yu, S.; Li, L. The effect of enzymatic pretreatment on subsequent octenyl succinic anhydride modification of corn starch. Food Hydrocoll. 2010, 24, 60–65. [Google Scholar] [CrossRef]
- Kim, H.-N.; Sandhu, K.S.; Lee, J.H.; Lim, H.S.; Lim, S.-T. Characterisation of 2-octen-1-ylsuccinylated waxy rice amylodextrins prepared by dry-heating. Food Chem. 2010, 119, 1189–1194. [Google Scholar] [CrossRef]
- Tizzotti, M.J.; Sweedman, M.C.; Tang, D.; Schaefer, C.; Gilbert, R.G. New1H NMR Procedure for the Characterization of Native and Modified Food-Grade Starches. J. Agric. Food Chem. 2011, 59, 6913–6919. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, L.; Xie, F.; Yu, L.; Li, B.; Xie, F. Preparation and characterisation of octenyl succinate starch as a delivery carrier for bioactive food components. Food Chem. 2011, 126, 1218–1225. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.-C.; Herrera, Á.; Prakash, O. Study of octenyl succinic anhydride-modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011, 83, 407–413. [Google Scholar] [CrossRef]
- Morros, J.; Levecke, B.; Infante, M.R. Hydrophobically modified inulin from alkenyl succinic anhydride in aqueous media. Carbohydr. Polym. 2011, 84, 1110–1116. [Google Scholar] [CrossRef]
- Kokubun, S.; Ratcliffe, I.; Williams, P.A. Synthesis, Characterization and Self-Assembly of Biosurfactants Based on Hydrophobically Modified Inulins. Biomacromolecules 2013, 14, 2830–2836. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsification properties of dodecenyl succinic anhydride derivatives of gum Arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Yang, L.; Qiu, D. Optimization of a synthesis procedure for octenyl succinic anhydride modified gum arabic. Food Sci. Biotechnol. 2015, 24, 7–13. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Sun, B.; Jin, Z.; Wu, S. Starch sodium dodecenyl succinate prepared by one-step extrusion and its properties. Carbohydr. Polym. 2015, 133, 90–93. [Google Scholar] [CrossRef]
- Thekkaepadil, V.V.; Senan, C.; Černík, M. Dodecenylsuccinic Anhydride Derivatives of Gum Karaya (Sterculia urens): Preparation, Characterization, and Their Antibacterial Properties. J. Agric. Food Chem. 2015, 63, 3757–3765. [Google Scholar]
- Shi, Y.; Li, C.; Zhang, L.; Huang, T.; Ma, D.; Tu, Z.-C.; Wang, H.; Xie, H.; Zhang, N.-H.; Ouyang, B.-L. Characterization and emulsifying properties of octenyl succinate anhydride modified Acacia seyal gum (gum arabic). Food Hydrocoll. 2017, 65, 10–16. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Van Der Meeren, P.; Hosseini, S.M.H. Study on hydrophobic modification of basil seed gum-based (BSG) films by octenyl succinate anhydride (OSA). Carbohydr. Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Soni, N.; Shah, N.N.; Singhal, R.S. Dodecenyl succinylated guar gum hydrolysate as a wall material for microencapsulation: Synthesis, characterization and evaluation. J. Food Eng. 2019, 242, 133–140. [Google Scholar] [CrossRef]
- Milliken Chemical Company. Available online: https://chemical.milliken.com/docs/default-source/default-document-library/specialty-chemical-anhydrides-flyer.pdf (accessed on 26 February 2020).
- Cheng, H.N.; Neiss, T.G. Solution NMR Spectroscopy of Food Polysaccharides. Polym. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Pitombeira, N.A.; Neto, J.G.V.; Silva, D.A.; Feitosa, J.P.; De Paula, H.C.; De Paula, H.C. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef] [PubMed]
- De Paula, H.C.; Heatley, F.; Budd, P.M. Characterization ofAnacardiumoccidentale exudate polysaccharide. Polym. Int. 1998, 45, 27–35. [Google Scholar] [CrossRef]
- Cunha, P.L.R.; Maciel, J.S.; Sierakowski, M.R.; DePaula, R.C.M.; Feitosa, J.P.A. Oxidation of cashew tree gum extrudate polysaccharide with TEMPO reagent. J. Braz. Chem. Soc. 2007, 18, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Feitosa, J.; Maciel, J.; De Paula, H.C.; DePaula, R. Characterization of crosslinked cashew gum derivatives. Carbohydr. Polym. 2006, 66, 16–26. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.-C.; Wetzel, D.L. Fourier Transform Infrared (FT-IR) Microspectroscopic Census of Single Starch Granules for Octenyl Succinate Ester Modification. J. Agric. Food Chem. 2009, 57, 6443–6448. [Google Scholar] [CrossRef]
- Wu, X.Y.; Lee, P.I. Preparation and characterization of inulin ester microspheres as drug carriers. J. Appl. Polym. Sci. 2000, 77, 833–840. [Google Scholar] [CrossRef]
- Khasbaatar, D.; Choi, U.S. Fourier Transform Infrared Spectroscopy Study on Cation adsorption on Viscose Rayon Succinate. Mong. J. Chem. 2014, 12, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Mothé, C.; Rao, M. Thermal behavior of gum arabic in comparison with cashew gum. Thermochim. Acta 2000, 357, 9–13. [Google Scholar] [CrossRef]
- Neto, É.D.M.; Maciel, J.D.S.; Cunha, P.L.R.; DePaula, R.C.M.; Feitosa, J.P. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J. Braz. Chem. Soc. 2011, 22, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.D.L.V.; Oliveira, A.C.D.J.; Filho, E.C.D.S.; Ribeiro, A.J.; Veiga, F.; Soares, M.F.D.L.R.; Wanderley, A.G.; Soares-Sobrinho, J.L. Nanostructured polymeric system based of cashew gum for oral admnistration of insulin. Matéria 2019, 24. [Google Scholar] [CrossRef]
- English, R.J.; Laurer, J.H.; Spontak, R.J.; Khan, S.A. Hydrophobically Modified Associative Polymer Solutions: Rheology and Microstructure in the Presence of Nonionic Surfactants. Ind. Eng. Chem. Res. 2002, 41, 6425–6435. [Google Scholar] [CrossRef]
- Blagodatskikh, I.; Vasil’Eva, O.; Ivanova, E.; Bykov, S.; Churochkina, N.; Pryakhina, T.; Smirnov, V.; Philippova, O.; Khokhlov, A. New approach to the molecular characterization of hydrophobically modified polyacrylamide. Polymer 2004, 45, 5897–5904. [Google Scholar] [CrossRef]










| Substrate | ASA used | Solvent used | Reference |
|---|---|---|---|
| corn starch | DDSA | water suspension, pH 8.5–9.0 | [27] |
| corn starch | OSA | pyridine | [28] |
| corn and amaranth starch | OSA | pyridine | [29] |
| corn starch | OSA | glacial acetic acid, 180 °C | [30] |
| corn starch | OSA | pyridine, DMSO, microwave | [31] |
| corn starch | DDSA | water suspension, pH 8.5 | [32] |
| corn starch | DDSA | starch film dipped in ethanol-diluted DDSA | [33] |
| cassava starch | OSA | water, microwave | [34] |
| corn starch | OSA | water suspension, pH 8.5 | [35] |
| rice amylodextrin | OSA | starch/OSA dissolved in water and dried; heated to 130–150 °C | [36] |
| corn starch | OSA | water suspension, pH 8.5 | [37] |
| corn starch | OSA | pyridine | [38] |
| corn starch | OSA | water suspension, pH 7.5–9.5 | [39] |
| inulin | OSA, DDSA | water suspension | [40] |
| inulin | OSA, DDSA | water dispersion, pH 8.5 | [41] |
| gum arabic | DDSA | water dispersion pH 8.3 | [42] |
| gum arabic | OSA | water dispersion | [43] |
| corn starch | DDSA | aqueous solution, extruded | [44] |
| gum karaya | DDSA | water dispersion, pH 8.5 | [45] |
| gum arabic | OSA | water dispersion, pH 8 | [46] |
| basil seed gum | OSA | water dispersion, pH 8 | [47] |
| guar gum | DDSA | water-NaHCO3 | [48] |
| cashew gum | OSA, TPSA | DMSO, 120 °C | this work |
| Sample | ASA used | Weight of ASA, g | Product weight, g | Yield % | Expected DS | Obsd DS |
|---|---|---|---|---|---|---|
| O1 | OSA | 0.02 | 0.955 | 94 | 0.015 | 0.021 |
| O2 | OSA | 0.04 | 0.972 | 93 | 0.031 | 0.035 |
| O3 | OSA | 0.084 | 1.054 | 97 | 0.065 | 0.063 |
| O4 | OSA | 0.104 | 1.039 | 94 | 0.080 | 0.074 |
| O5 | OSA | 0.2 | 1.063 | 89 | 0.154 | 0.155 |
| T1 | TPSA | 0.02 | 0.930 | 91 | 0.012 | 0.012 |
| T2 | TPSA | 0.04 | 0.941 | 90 | 0.024 | 0.024 |
| T3 | TPSA | 0.084 | 1.034 | 95 | 0.051 | 0.048 |
| T4 | TPSA | 0.104 | 0.987 | 89 | 0.063 | 0.062 |
| T5 | TPSA | 0.2 | 1.050 | 88 | 0.122 | 0.113 |
| Sample | ASA used | Mw (kDa) | Mn (kDa) | Mw/Mn |
|---|---|---|---|---|
| CG control | none | 31.7 | 18.2 | 1.74 |
| O1 | OSA | 28.5 | 16.3 | 1.75 |
| O2 | OSA | 28.1 | 15.4 | 1.83 |
| O3 | OSA | 23.2 | 12.5 | 1.86 |
| O4 | OSA | 21.4 | 11.3 | 1.89 |
| O5 | OSA | 10.0 | 6.1 | 1.64 |
| T1 | TPSA | 26.1 | 14.4 | 1.81 |
| T2 | TPSA | 22.4 | 11.7 | 1.91 |
| T3 | TPSA | 14.4 | 7.3 | 1.97 |
| T4 | TPSA | 13.2 | 6.8 | 1.94 |
| T5 | TPSA | 12.3 | 5.4 | 2.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, A.; Cheng, H.N.; Kim, S.; Alves, C.R.; Furtado, R.F. Hydrophobic Modification of Cashew Gum with Alkenyl Succinic Anhydride. Polymers 2020, 12, 514. https://doi.org/10.3390/polym12030514
Biswas A, Cheng HN, Kim S, Alves CR, Furtado RF. Hydrophobic Modification of Cashew Gum with Alkenyl Succinic Anhydride. Polymers. 2020; 12(3):514. https://doi.org/10.3390/polym12030514
Chicago/Turabian StyleBiswas, Atanu, H. N. Cheng, Sanghoon Kim, Carlucio R. Alves, and Roselayne F. Furtado. 2020. "Hydrophobic Modification of Cashew Gum with Alkenyl Succinic Anhydride" Polymers 12, no. 3: 514. https://doi.org/10.3390/polym12030514