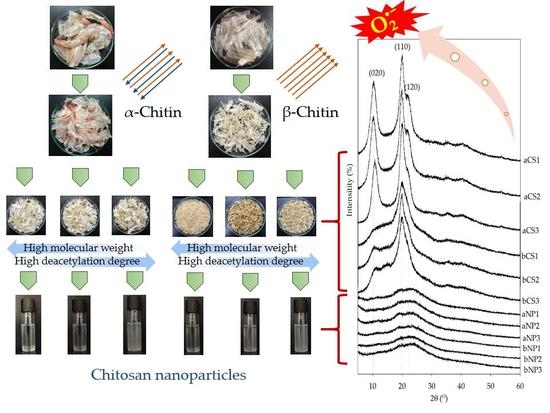
Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitin and Chitosan
2.3. Preparation of Chitosan Nanoparticles
2.4. Characterisation of Chitosan and Chitosan Nanoparticles
2.4.1. %DD
2.4.2. MW
2.4.3. X-ray Diffraction
2.4.4. Particle Size, Polydispersity Index (PDI), and Zeta Potential
2.5. Superoxide Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Chitosan and Chitosan Nanoparticles
3.1.1. %DD and MW of chitosan
3.1.2. Crystalline Structure of Chitosan and Chitosan Nanoparticles
3.1.3. Particle Size, Polydispersity Index (PDI) and Zeta Potential of Chitosan Nanoparticles
3.2. Superoxide Radical Scavenging Activities of Chitosan and Chitosan Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 208, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Mahdy Samar, M.; El-Kalyoubi, M.H.; Khalaf, M.M.; Abd El-Razik, M.M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, Y.; Bao, E.; Zhao, Y. Preparation, characterization and toxicology properties of alpha- and beta-chitosan Maillard reaction products nanoparticles. Int. J. Biol. Macromol. 2016, 89, 287–296. [Google Scholar] [CrossRef]
- Subhapradha, N.; Ramasamy, P.; Shanmugam, V.; Madeswaran, P.; Srinivasan, A.; Shanmugam, A. Physicochemical characterisation of β-chitosan from Sepioteuthis lessoniana gladius. Food Chem. 2013, 141, 907–913. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Impact of the structural differences between alpha- and beta-chitosan on their depolymerizing reaction and antibacterial activity. J. Agric. Food Chem. 2013, 61, 8783–8789. [Google Scholar] [CrossRef]
- Jung, J.; Zhao, Y. Comparison in antioxidant action between alpha-chitosan and beta-chitosan at a wide range of molecular weight and chitosan concentration. Bioorg. Med. Chem. 2012, 20, 2905–2911. [Google Scholar] [CrossRef]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, R.; Wang, Y.; Li, M.; Zhu, Y.; Chen, L.; Kang, L.; Liu, Z.; Yang, Y.; Yuan, S. A novel thermophilic exochitinase ChiEn3 from Coprinopsis cinerea exhibits a hyperhydrolytic activity toward 85% deacetylated chitosan and a significant application to preparation of chitooligosaccharides from the chitosan. Carbohydr. Polym. 2019, 207, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliyahu, S.; Aharon, A.; Bianco-Peled, H. Acrylated Chitosan Nanoparticles with Enhanced Mucoadhesion. Polymers 2018, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Zhang, T.; Yu, Y.; Zhang, Y.; Zhai, J.; Huang, H.; Wei, S.; Ding, L.; Liu, B. A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem. 2019, 286, 530–536. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Alhajj, N.; Zakaria, Z.; Naharudin, I.; Ahsan, F.; Li, W.; Wong, T.W. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG 3000 microparticles in pulmonary inhalation. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Chachuli, S.H.; Nawaz, A.; Shah, K.; Naharudin, I.; Wong, T.W. In Vitro Investigation of Influences of Chitosan Nanoparticles on Fluorescein Permeation into Alveolar Macrophages. Pharm. Res. 2016, 33, 1497–1508. [Google Scholar] [CrossRef]
- Javaid, M.A.; Rizwan, M.; Khera, R.A.; Zia, K.M.; Saito, K.; Zuber, M.; Iqbal, J.; Langer, P. Thermal degradation behavior and X-ray diffraction studies of chitosan based polyurethane bio-nanocomposites using different diisocyanates. Int. J. Biol. Macromol. 2018, 117, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yui, T.; Miya, M. Dependence on the Preparation Procedure of the Polymorphism and Crystallinity of Chitosan Membranes. Biosci. Biotechnol. Biochem. 1992, 56, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Muley, A.B.; Chaudhari, S.A.; Mulchandani, K.H.; Singhal, R.S. Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int. J. Biol. Macromol. 2018, 111, 1047–1058. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.I.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Divya, K.; Smitha, V.; Jisha, M.S. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018, 114, 572–577. [Google Scholar] [CrossRef]
- Anusha, J.R.; Fleming, A.T.; Valan Arasu, M.; Chul Kim, B.; Al-Dhabi, N.A.; Yu, K.H.; Justin Raj, C. Mechanochemical synthesis of chitosan submicron particles from the gladius of Todarodes pacificus. J. Adv. Res. 2016, 7, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Kiang, T.; Wen, J.; Lim, H.W.; Leong, K.W. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials 2004, 25, 5293–5301. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Wang, P.; Li, C.; Li, Z.; Li, P. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorg. Med. Chem. 2005, 13, 1573–1577. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Designing chitosan-tripolyphosphate microparticles with desired size for specific pharmaceutical or forensic applications. Int. J. Biol. Macromol. 2017, 95, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Ku, M.-W.; Chen, P.-W. Extraction of crude chitosans from squid (Illex argentinus) pen by a compressional puffing-pretreatment process and evaluation of their antibacterial activity. Food Chem. 2018, 254, 217–223. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xing, R.; Li, K.; Qin, Y.; Zou, P.; Liu, S.; Yu, H.; Li, P. Beta-chitosan extracted from Loligo Japonica for a potential use to inhibit Newcastle disease. Int. J. Biol. Macromol. 2016, 82, 614–620. [Google Scholar] [CrossRef]
- de Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocolloids 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wei, L.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antioxidant Evaluation of Novel Pyridylurea-Functionalized Chitosan Derivatives. Polymers 2019, 11, 951. [Google Scholar] [CrossRef] [Green Version]
- Je, J.Y.; Kim, S.K. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg. Med. Chem. 2006, 14, 5989–5994. [Google Scholar] [CrossRef]
- Arata Badano, J.; Vanden Braber, N.; Rossi, Y.; Díaz Vergara, L.; Bohl, L.; Porporatto, C.; Falcone, R.D.; Montenegro, M. Physicochemical, in vitro antioxidant and cytotoxic properties of water-soluble chitosan-lactose derivatives. Carbohydr. Polym. 2019, 224, 115158. [Google Scholar] [CrossRef] [PubMed]








| Samples | CI | (020) | (110) | (120) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | 2θ(°) | d (Å) | RI (%) | 2θ(°) | d (Å) | RI (%) | 2θ(°) | d (Å) | RI (%) | |
| aCS1 | 63.12 ± 1.67c | 10.2 | 8.63 | 80 | 20.1 | 4.42 | 100 | 21.9 | 4.05 | 68 |
| aCS2 | 68.04 ± 0.89b | 10.3 | 8.53 | 77 | 20.0 | 4.43 | 100 | 21.8 | 4.07 | 65 |
| aCS3 | 70.63 ± 0.71a | 10.6 | 8.28 | 68 | 19.9 | 4.46 | 100 | 21.8 | 4.08 | 61 |
| bCS1 | 48.42 ± 1.82f | 10.2 | 8.64 | 44 | 19.9 | 4.46 | 100 | - | - | - |
| bCS2 | 55.81 ± 1.32d | 10.6 | 8.33 | 44 | 19.9 | 4.46 | 100 | 21.8 | 4.08 | 70 |
| bCS3 | 52.13 ± 0.86e | 11.3 | 7.86 | 46 | 20.0 | 4.44 | 100 | 21.1 | 4.20 | 80 |
| aNP1 | 23.63 ± 1.23g | 11.7 | 7.55 | 73 | 19.4 | 4.58 | 95 | 22.6 | 3.94 | 100 |
| aNP2 | 19.55 ± 1.15h | 12.0 | 7.38 | 75 | 19.84 | 4.47 | 99 | 22.4 | 3.96 | 100 |
| aNP3 | 23.70 ± 2.73g | 11.8 | 7.49 | 76 | 19.1 | 4.65 | 98 | 22.6 | 3.93 | 100 |
| bNP1 | 21.36 ± 1.59gh | 9.2 | 9.55 | 69 | 19.0 | 4.67 | 97 | 22.0 | 4.03 | 100 |
| bNP2 | 22.70 ± 0.37g | 12.2 | 7.26 | 74 | 19.8 | 4.49 | 97 | 22.6 | 3.93 | 100 |
| bNP3 | 23.74 ± 2.29g | 12.7 | 6.97 | 74 | 19.8 | 4.48 | 99 | 22.6 | 3.93 | 100 |
| Samples | Particles Size (nm) | PDI * | Zeta Potential (mV) |
|---|---|---|---|
| aNP1 | 169.23 ± 3.33d | 0.314 ± 0.015a | 23.07 ± 1.15c |
| aNP2 | 165.03 ± 2.57d | 0.271 ± 0.014a | 24.43 ± 0.81bc |
| aNP3 | 332.20 ± 15.85a | 0.168 ± 0.101bc | 26.33 ± 2.91b |
| bNP1 | 192.50 ± 1.91c | 0.248 ± 0.012ab | 24.83 ± 0.49bc |
| bNP2 | 170.13 ± 2.29d | 0.152 ± 0.011c | 25.40 ± 0.56bc |
| bNP3 | 232.30 ± 3.27b | 0.098 ± 0.052c | 30.57 ± 0.64a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. https://doi.org/10.3390/polym11122010
Jampafuang Y, Tongta A, Waiprib Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers. 2019; 11(12):2010. https://doi.org/10.3390/polym11122010
Chicago/Turabian StyleJampafuang, Yattra, Anan Tongta, and Yaowapha Waiprib. 2019. "Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities" Polymers 11, no. 12: 2010. https://doi.org/10.3390/polym11122010