Synergistic Effect of Maleated Natural Rubber and Modified Palm Stearin as Dual Compatibilizers in Composites based on Natural Rubber and Halloysite Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MPS
2.3. Synthesis of MPS
2.4. Preparation of Composites based on NR and HNT
2.5. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.6. Determination of Curing Characteristics
2.7. Measurement of Mechanical Properties
2.8. Dynamic Properties
2.9. Scanning Electron Microscopy
3. Results and Discussion
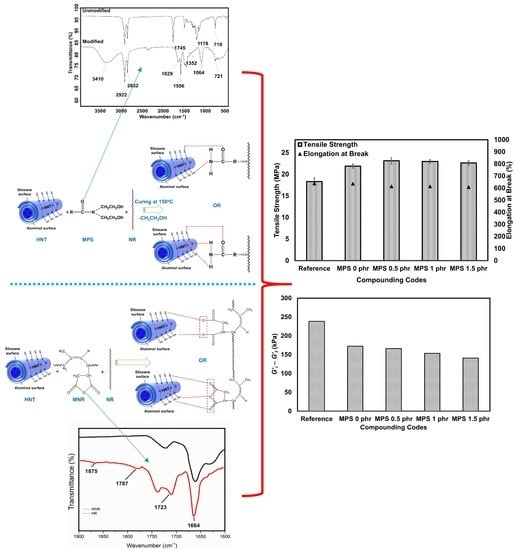
3.1. Functionalities of Maleated Natural Rubber
3.2. Functionalities of Maleated Natural Rubber
3.3. Cure Characteristics
3.4. Mechanical Properties
3.5. Dynamic Properties
3.6. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arrighi, V.; McEwen, I.; Qian, H.; Prieto, M.S. The glass transition and interfacial layer in styrene-butadiene rubber containing silica nanofiller. Polymers 2003, 44, 6259–6266. [Google Scholar] [CrossRef]
- Jovanović, V.; Samaržija-Jovanović, S.; Marković, G.; Marinović-Cincović, M.; Budinski-Simendić, J. Mechanical and Morphological Properties Rubber blends reinforced with nanofillers. KGK Kautschuk Gummi Kunststoffe 2011, 9, 52–56. [Google Scholar]
- Chakravarty, S.; Chakravarty, A. Reinforcement of rubber compounds with nano-filler. KGK Kautschuk Gummi Kunststoffe 2007, 60, 619–622. [Google Scholar]
- Hu, D.; Zhang, Z.; Liu, M.; Lin, J.; Chen, X.; Ma, W. Multifunctional UV-shielding nanocellulose films modified with halloysite nanotubes-zinc oxide nanohybrid. Cellulose 2020, 27, 401–413. [Google Scholar] [CrossRef]
- Cavallaro, G.; Chiappisi, L.; Pasbakhsh, P.; Gradzielski, M.; Lazzara, G. A structural comparison of halloysite nanotubes of different origin by Small-Angle Neutron Scattering (SANS) and Electric Birefringence. Appl. Clay Sci. 2018, 160, 71–80. [Google Scholar] [CrossRef]
- Feng, K.; Hung, G.-Y.; Liu, J.; Li, M.; Zhou, C.; Liu, M. Fabrication of high performance superhydrophobic coatings by spray-coating of polysiloxane modified halloysite nanotubes. Chem. Eng. J. 2018, 331, 744–754. [Google Scholar] [CrossRef]
- Hu, D.; Zhong, B.; Jia, Z.; Lin, J.; Liu, M.; Luo, Y.; Jia, D. A novel hybrid filler of halloysite nanotubes/silica fabricated by electrostatic self-assembly. Mater. Lett. 2017, 188, 327–330. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Varghese, S.; Karger-Kocsis, J. Natural rubber-based nanocomposites by latex compounding with layered silicates. Polymers 2003, 44, 4921–4927. [Google Scholar] [CrossRef]
- Paran, S.; Naderi, G.; Ghoreishy, M. XNBR-grafted halloysite nanotube core-shell as a potential compatibilizer for immiscible polymer systems. Appl. Surf. Sci. 2016, 382, 63–72. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Ismail, H.; Fauzi, M.A.; Bakar, A.A. Influence of maleic anhydride grafted ethylene propylene diene monomer (MAH-g-EPDM) on the properties of EPDM nanocomposites reinforced by halloysite nanotubes. Polym. Test. 2009, 28, 548–559. [Google Scholar] [CrossRef]
- Norizzah, A.R.; Chong, C.; Cheow, C.; Zaliha, O. Effects of chemical interesterification on physicochemical properties of palm stearin and palm kernel olein blends. Food Chem. 2004, 86, 229–235. [Google Scholar] [CrossRef]
- Che Man, Y.; Haryati, T.; Ghazali, H.; Asbi, B. Composition and thermal profile of crude palm oil and its products. J. Am. Oil Chem. Soc. 1999, 76, 237–242. [Google Scholar] [CrossRef]
- Surya, I.; Ismail, H.; Azura, A. Alkanolamide as an accelerator, filler-dispersant and a plasticizer in silica-filled natural rubber compounds. Polym. Test. 2013, 32, 1313–1321. [Google Scholar] [CrossRef]
- Kolancilar, H. Preparation of laurel oil alkanolamide from laurel oil. J. Am. Oil Chem. Soc. 2004, 81, 597–598. [Google Scholar] [CrossRef]
- Surya, I.; Ismail, H. The effect of the addition of alkanolamide on properties of carbon black-filled natural rubber (SMR-L) compounds cured using various curing systems. Polym. Test. 2016, 50, 276–282. [Google Scholar] [CrossRef]
- Bhargava, R.; Wang, S.-Q.; Koenig, J.L. FTIR Microspectroscopy of Polymeric Systems, Liquid Chromatography/FTIR Microspectroscopy/Microwave Assisted Synthesis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 137–191. [Google Scholar]
- Nakason, C.; Kaesaman, A.; Supasanthitikul, P. The grafting of maleic anhydride onto natural rubber. Polym. Test. 2004, 23, 35–41. [Google Scholar] [CrossRef]
- Sahakaro, K.; Beraheng, S. Reinforcement of maleated natural rubber by precipitated silica. J. Appl. Polym. Sci. 2008, 109, 3839–3848. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Coran, A. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Payne, A.; Whittaker, R. Low strain dynamic properties of filled rubbers. Rubb. Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Cooperative effects of epoxide functional groups on natural rubber and silane coupling agents on reinforcing efficiency of silica. Rubb. Chem. Technol. 2014, 87, 291–310. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wang, D.-Y.; Galiatsatos, V.; Heinrich, G. Understanding the reinforcing behavior of expanded clay particles in natural rubber compounds. Soft Matter 2013, 9, 3798–3808. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A. Recycled polyethylene terephthalate filled natural rubber compounds: Effects of filler loading and types of matrix. J. Elast. Plast. 2011, 43, 429–449. [Google Scholar] [CrossRef]
- Waesateh, K.; Saiwari, S.; Ismail, H.; Othman, N.; Soontaranon, S.; Hayeemasae, N. Features of crystallization behavior of natural rubber/halloysite nanotubes composites using synchrotron wide-angle X-ray scattering. Int. J. Polym. Anal. Charac. 2018, 23, 260–270. [Google Scholar] [CrossRef]













| Ingredient | Compounding Code and Amounts in phr | ||||
|---|---|---|---|---|---|
| Reference | MPS 0 phr | MPS 0.5 phr | MPS 1.0 phr | MPS 1.5 phr | |
| NR | 100 | 90 | 90 | 90 | 90 |
| MNR * | - | 10 | 10 | 10 | 10 |
| ZnO | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | 1 | 1 | 1 | 1 | 1 |
| CBS | 2 | 2 | 2 | 2 | 2 |
| Sulfur | 2 | 2 | 2 | 2 | 2 |
| HNT | 10 | 10 | 10 | 10 | 10 |
| MPS | - | - | 0.5 | 1 | 1.5 |
| Wavenumber (cm-1) | Assignment |
|---|---|
| 3410 | O–H stretch |
| 2922, 2852 | C–H stretch |
| 1745 | C=O stretch in ester |
| 1629, 1556 | C=O stretch in a modified structure |
| 1352 | CH3 umbrella mode |
| 1064 | C–N stretch |
| 719/721 | CH2 rocking |
| Wavenumber cm−1 | Assignment |
|---|---|
| 2900 | C–H stretch of NR |
| 1875 | C=O stretch of succinic anhydride (weak) |
| 1787 | C=O stretch of polymeric anhydride (weak) |
| 1723 | C=O stretch of a carbonyl group |
| 1664 | C=C stretch of NR |
| 835 | C–H out of plane bend of NR |
| Compound Code | MH (dN.m) | MH − ML (dN.m) | tS1 (min) | tc90 (min) |
|---|---|---|---|---|
| Reference | 7.43 | 7.18 | 0.84 | 2.81 |
| MPS 0 phr | 7.33 | 6.38 | 1.25 | 3.16 |
| MPS 0.5 phr | 9.27 | 8.35 | 0.7 | 2.84 |
| MPS 1.0 phr | 9.32 | 8.37 | 0.64 | 2.76 |
| MPS 1.5 phr | 9.1 | 8.2 | 0.56 | 2.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayeemasae, N.; Sensem, Z.; Surya, I.; Sahakaro, K.; Ismail, H. Synergistic Effect of Maleated Natural Rubber and Modified Palm Stearin as Dual Compatibilizers in Composites based on Natural Rubber and Halloysite Nanotubes. Polymers 2020, 12, 766. https://doi.org/10.3390/polym12040766
Hayeemasae N, Sensem Z, Surya I, Sahakaro K, Ismail H. Synergistic Effect of Maleated Natural Rubber and Modified Palm Stearin as Dual Compatibilizers in Composites based on Natural Rubber and Halloysite Nanotubes. Polymers. 2020; 12(4):766. https://doi.org/10.3390/polym12040766
Chicago/Turabian StyleHayeemasae, Nabil, Zareedan Sensem, Indra Surya, Kannika Sahakaro, and Hanafi Ismail. 2020. "Synergistic Effect of Maleated Natural Rubber and Modified Palm Stearin as Dual Compatibilizers in Composites based on Natural Rubber and Halloysite Nanotubes" Polymers 12, no. 4: 766. https://doi.org/10.3390/polym12040766