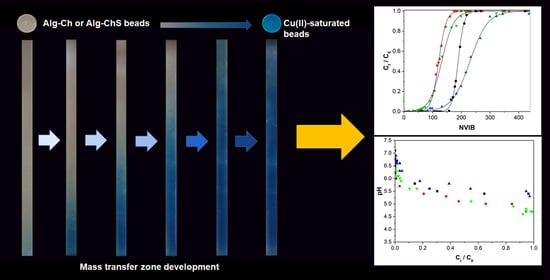
Removal of Cu(II) by Fixed-Bed Columns Using Alg-Ch and Alg-ChS Hydrogel Beads: Effect of Operating Conditions on the Mass Transfer Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Chitosan and Chitosan Sulfate
2.3. Preparation of Ch (Alg-Ch) and Alginate-ChS (Alg-ChS) Beads
2.4. Beads Characterization
2.5. Adsorption Equilibrium
2.6. Fixed Bed Column Studies on Copper(II) Adsorption from Aqueous Solution
2.7. Column Data Analysis
2.8. Fixed Bed Model
3. Results and Discussion
3.1. Characterization of Ch and ChS
3.1.1. FTIR Spectroscopy
3.1.2. Chemical Elementary Analysis of ChS
3.1.3. Potentiometric Titration of Ch and ChS
3.2. Characterization of Hydrogel Beads
3.2.1. Hydrogel Bead Composition
3.2.2. Average Diameter and Morphology of the Hydrogel Beads
3.3. Adsorption Equilibrium
3.4. Adsorption Studies in Fixed-Bed Columns
3.4.1. Flow Effect
3.4.2. Effect of Bed Height
3.4.3. Effect of Copper(II) Ion Concentration
3.4.4. pH Effect
3.5. Modeling of the Behavior of the Fixed-Bed Column
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Dichiara, A.B.; Weinstein, S.; Rogers, R.E. On the Choice of Batch or Fixed-Bed Adsorption Processes for Wastewater Treatment. Ind. Eng. Chem. Res. 2015, 54, 8579–8586. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan Ngah, W.S.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Sekar, S.; Surianarayanan, M.; Ranganathan, V.; MacFarlane, D.R.; Mandal, A.B. Choline-based ionic liquids-enhanced biodegradation of azo dyes. Environ. Sci. Technol. 2012, 46, 4902–4908. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhay, M.; Pal, A. Fixed bed column study for the removal of crystal violet (C.I. Basic Violet 3) dye from aquatic environment by surfactant-modified alumina. Dye. Pigment. 2006, 245–251. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Cussler, E.L. Adsorption. In Diffusion; Cambridge University Press: Cambridge, UK, 2009; pp. 375–403. [Google Scholar]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin—And chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of Chitosan for the Removal of Metals from Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Flores-Alamo, N.; Solache-Rios, M.J.; Gómez-Espinoza, R.M.; García Gaytan, B. Estudio de adsorción competitiva de cobre y zinc en solución acuosa utilizando Q/PVA/EGDE. Rev. Mex. Ing. Qum. 2015, 14, 801–811. [Google Scholar]
- García-González, R.; Gómez-Espinosa, R.M.; Ávila-Pérez, P.; Garcia-Gaitán, B.; García-Rivas, J.L.; Zavala-Arce, R.E. Estudio de biosorción de Cu2+ en el criogel quitosano-celulosa. Rev. Mex. Ing. Quím. 2016, 15, 311–322. [Google Scholar]
- Li, B.; Zhou, F.; Huang, K.; Wang, Y.; Mei, S.; Zhou, Y.; Jing, T. Environmentally friendly chitosan/PEI-grafted magnetic gelatin for the highly effective removal of heavy metals from drinking water. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mone, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G. Chitosan grafted with biobased 5-hydroxymethyl-furfural as adsorbent for copper and cadmium ions removal. Polymers 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Balleño, A.; Aranda-García, F.; Morales, J.A.; Mendizábal, E.; Katime, I. Alginate-chitosan and alginate-chitosan sulfate hydrogels for the removal of copper ions. Rev. Iberoam. Polímeros 2016, 17, 255–265. [Google Scholar]
- Ríos-Donato, N.; Peña-Flores, A.M.; Katime, I.; Leyva-Ramos, R.; Mendizábal, E. Kinetics and thermodynamics of adsorption of red dye 40 from acidic aqueous solutions onto a novel chitosan sulfate. Afinidad 2017, 74, 214–220. [Google Scholar]
- Qin, Y.M.; Cai, L.L.; Feng, D.M.; Shi, B.B.; Liu, J.J.; Zhang, W.T.; Shen, Y.C. Combined use of chitosan and alginate in the treatment of wastewater. J. Appl. Polym. Sci. 2007, 104, 3581–3587. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal—Carboxylate interactions in metal—Alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Veglio, F.; Esposito, A.; Reverberi, A.P. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar] [CrossRef]
- Aksu, Z.; Cagatay, S.S.; Gönen, F. Continuous fixed bed biosorption of reactive dyes by dried Rhizopus arrhizus: Determination of column capacity. J. Hazard. Mater. 2007, 143, 362–371. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rouf, S.; Nagapadma, M. Modeling of Fixed Bed Column Studies for Adsorption of Azo Dye on Chitosan Impregnated with a Cationic Surfactant. Int. J. Sci. Eng. Res. 2015, 6, 538–545. [Google Scholar] [CrossRef]
- Vieira, M.L.G.; Esquerdo, V.M.; Nobre, L.R.; Dotto, G.L.; Pinto, L.A.A. Glass beads coated with chitosan for the food azo dyes adsorption in a fixed bed column. J. Ind. Eng. Chem. 2014, 20, 3387–3393. [Google Scholar] [CrossRef]
- Ríos Donato, N.; Carrión Espinoza, L.G.; Mayorga Rivera, J.A.; Verduzco Navarro, I.P.; Katime, I.; Mendizábal, E. Removal of Cd (II) from aqueous solutions by batch and continuous process using chitosan sulfate dispersed in a calcium alginate hydrogel. AFINIDAD 2018, 75, 112–118. [Google Scholar]
- Geankoplis, C.; Hersel, A.; Lepek, D. Transport Processes and Separation Process Principles; Pearson Eduaction: Upper Saddle River, NJ, USA, 2018; ISBN 9780134181028. [Google Scholar]
- Barros, M.A.S.D.; Arroyo, P.A.; Silva, E.A. General Aspects of Aqueous Sorption Process in Fixed Beds. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; Nakajima, H., Ed.; IntechOpen: London, UK, 2013; pp. 361–386. [Google Scholar]
- Verduzco-Navarro, I.P.; Ríos-Donato, N.; Mendizabal, E.; Katime, I. Red dye 40 removal using alginate-chitosan and alginate-chitosan sulfate beads. Rev. Ciencias Ambient. Recur. Nat. 2016, 2, 33–43. [Google Scholar]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mondal, S.; De, S. Design and scaling up of fixed bed adsorption columns for lead removal by treated laterite. J. Clean. Prod. 2018, 177, 760–774. [Google Scholar] [CrossRef]
- Naja, G.; Volesky, B. Behavior of the mass transfer zone in a biosorption column. Environ. Sci. Technol. 2006, 40, 3996–4003. [Google Scholar] [CrossRef]
- Tien, C. Fixed-Bed Adsorption Models and Fixed-Bed Design Calculations. In Introduction to Adsorption; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–199. ISBN 9780128164464. [Google Scholar]
- Dotto, G.L.; Gonçalves Salau, N.P.; Piccin, J.S.; Sant’Anna Cadaval, T.R.J.; Almeida de Pinto, L.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petricolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 53–74. [Google Scholar]
- Saha, T.K. Adsorption of Methyl Orange onto Chitosan from Aqueous Solution. J. Water Resour. Prot. 2010, 2, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Teophanides, T., Ed.; InTechOpen: London, UK, 2012; pp. 953–978. ISBN 978-953-51-0537-4. [Google Scholar]
- Ríos Donato, N.; Navarro, R.; Avila Rodríguez, M.; Mendizábal, E. Coagulation–flocculation of colloidal suspensions of kaolinite, bentonite, and alumina by chitosan sulfate. J. Appl. Polym. Sci. 2012, 123, 2003–2010. [Google Scholar] [CrossRef]
- Wankat, P.C. Separation Process Engineering, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2008; ISBN 9780131382275. [Google Scholar]
- Xu, Z.; Cai, J.G.; Pan, B.C. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univ. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Hasfalina, C.M.; Maryam, R.Z.; Luqman, C.A.; Rashid, M. Adsorption of Copper (II) From Aqueous Medium in Fixed-Bed Column by Kenaf Fibres. APCBEE Procedia 2012, 3, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Kapur, M.; Mondal, M.K. Design and model parameters estimation for fixed–bed column adsorption of Cu(II) and Ni(II) ions using magnetized saw dust. Desalin. Water Treat. 2016, 57, 12192–12203. [Google Scholar] [CrossRef]
- Liu, D.; Sun, D. Modeling Adsorption of Cu(II) Using Polyaniline-Coated Sawdust in a Fixed-Bed Column. Environ. Eng. Sci. 2012, 29, 461–465. [Google Scholar] [CrossRef]
- Chen, J.-D.; Yu, J.X.; Wang, F.; Tang, J.-Q.; Zhang, Y.-F.; Xu, Y.-L.; Chi, R.-A. Selective adsorption and recycle of Cu2+ from aqueous solution by modified sugarcane bagasse under dynamic condition. Environ. Sci. Pollut. Res. 2017, 24, 9202–9209. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Vázquez, S.I.; Vidales-Contreras, J.A.; Márquez-Reyes, J.M.; Cruz-López, A.; García-Gómez, C. Removal of congo red dye using electrocoagulated metal hydroxide in a fixed-bed column: Characterization, optimization and modeling studies. Rev. Mex. Ing. Quím. 2019, 18, 1133–1142. [Google Scholar] [CrossRef]
- Radnia, H.; Ghoreyshi, A.A.; Younesi, H.; Masomi, M.; Pirzadeh, K. Adsorption of Fe(II) from aqueous phase by chitosan: Application of physical models and artificial neural network for prediction of breakthrough. Int. J. Eng. Trans. B Appl. 2013, 26, 845–858. [Google Scholar] [CrossRef]
- Dhoble, R.M.; Maddigapu, P.R.; Rayalu, S.S.; Bhole, A.G.; Dhoble, A.S.; Dhoble, S.R. Removal of arsenic(III) from water by magnetic binary oxide particles (MBOP): Experimental studies on fixed bed column. J. Hazard. Mater. 2017, 322, 469–478. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Pascua, C.; Wan, M.W. Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 697–704. [Google Scholar] [CrossRef]
- Al-Remawi, M.M.A. Properties of chitosan nanoparticles formed using sulfate anions as crosslinking bridges. Am. J. Appl. Sci. 2012, 9, 1091–1100. [Google Scholar]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.A.; Tamer, T.M.; Abd Elmonem, M.S.; Ibrahim, S.A. Novel smart pH sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: I. Preparation and characterization. Int. J. Pharm. Pharm. Sci. 2015, 7, 320–326. [Google Scholar]
- Mangaleshwaran, L.; Thirulogachandar, A.; Rajasekar, V.; Muthukumaran, C.; Rasappan, K. Batch and fixed bed column studies on nickel (II) adsorption from aqueous solution by treated polyurethane foam. J. Taiwan Inst. Chem. Eng. 2015, 55, 112–118. [Google Scholar] [CrossRef]
- Silva, P.M.O.; Francisco, J.E.; Cajé, J.C.M.; Cassella, R.J.; Pacheco, W.F. A batch and fixed bed column study for fluorescein removal using chitosan modified by epichlorohydrin. J. Environ. Sci. Heal. Part A Toxic Hazard. Subst. Environ. Eng. 2018, 53, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mishra, U. Continuous Fixed-Bed Column Study and Adsorption Modeling: Removal of Lead Ion from Aqueous Solution by Charcoal Originated from Chemical Carbonization of Rubber Wood Sawdust. J. Chem. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Verduzco-Navarro, I.P.; Jasso-Gastinel, C.F.; Ríos-Donato, N.; Mendizábal, E. Red dye 40 removal by fixed-bed columns packed with alginate-chitosan sulfate hydrogels. Rev. Mex. Ing. Quím. 2020, 19, 1401–1411. [Google Scholar] [CrossRef]
- Xia, K.; Liu, X.; Wang, W.; Yang, X.; Zhang, X. Synthesis of modified starch/polyvinyl alcohol composite for treating textile wastewater. Polymers 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Verduzco-Navarro, I.P.; Álvarez-Vázquez, D.; Yáñez-Barragán, S.I.; Jasso-Gastinel, C.F.; Ríos-Donato, N.; Mendizábal, E. Red dye 40 removal by percolation using columns packed with chitosan-alginate hydrogels. Rev. Iberoam. Polímeros 2018, 20, 246–254. [Google Scholar]
- Hasanzadeh, M.; Ansari, R.; Ostovar, F. Synthesis and application of CeO2/sawdust nanocomposite for removal of As(III) ions from aqueous solutions using a fixed bed column system. Glob. Nest J. 2017, 19, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P. Das Artificial neural network (ANN) modeling of dynamic adsorption of crystal violet from aqueous solution using citric-acid-modified rice (Oryza sativa) straw as adsorbent. Clean Technol. Environ. Policy 2013, 15, 255–264. [Google Scholar] [CrossRef]
- Thakur, B.; Biswas, M.; Bandyopadhyay, K.; Misra, A.K. Fixed Bed Column Adsorption Studies Using Fly Ash for Removal of Cadmium from Aqueous Solution. J. Inst. Eng. India Ser. A 2014, 94, 161–168. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Zou, S.W.; Chen, J.P. Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study. Environ. Sci. Technol. 2008, 42, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Che-Galicia, G.; Martínez-Vera, C.; Ruiz-Martínez, R.S.; Castillo-Araiza, C.O. Modelling of a fixed bed adsorber based on an isotherm model or an apparent kinetic model. Rev. Mex. Ing. Quim. 2014, 13, 539–553. [Google Scholar]
- Zhou, D.; Zhang, L.; Zhou, J.; Guo, S. Development of a fixed-bed column with cellulose/chitin beads to remove heavy-metal ions. J. Appl. Polym. Sci. 2004, 94, 684–691. [Google Scholar] [CrossRef]









| Alg-Ch | Alg-ChS | ||
|---|---|---|---|
| Ce (mg/L) | qe (mg/g xerogel) | Ce (mg/L) | qe (mg/g xerogel) |
| 2.76 | 32.1 | 8.5 | 29.2 |
| 5.99 | 65.1 | 24.4 | 56.1 |
| 43.6 | 95.4 | 126.1 | 87.5 |
| 168.7 | 121.5 | 253.5 | 110.6 |
| 274.6 | 177.3 | 364.1 | 139.2 |
| 465.4 | 179.3 | 503.2 | 158.6 |
| Alg-Ch | Alg-ChS | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | qm (mg/g) | KL (L/mg) | R2 |
| 186 | 0.0201 | 0.930 | 170 | 0.013 | 0.960 |
| Hydrogel Type | C0 mg/L | Flow rate (mL/h) | HT cm | HL (cm) | HLUB (cm) | tb (h) | te (h) | NIVB 1 at tb | qb (mg Cu2+/g xerogel) | qe (mg Cu2+/g−1 xerogel) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alg-Ch | 50 | 50 | 13.0 | 6.4 | 6.6 | 20.2 | 62.6 | 111.8 | 48.3 | 99.5 |
| 50 | 100 | 13.0 | 4.0 | 9.0 | 6.6 | 32.0 | 73.9 | 28.9 | 94.6 | |
| 100 | 100 | 13.0 | 4.4 | 8.6 | 3.5 | 25.3 | 38.7 | 29.8 | 88.0 | |
| 50 | 50 | 33.0 | 27.6 | 5.4 | 71.9 | 100.2 | 157.3 | 85.1 | 101.6 | |
| Alg-ChS | 50 | 50 | 13.0 | 6.8 | 6.2 | 12.1 | 38.2 | 70.6 | 45.2 | 87.0 |
| 50 | 100 | 13.0 | 3.7 | 9.3 | 3.9 | 36.1 | 43.8 | 25.3 | 89.6 | |
| 100 | 100 | 13.0 | 3.6 | 9.4 | 2.0 | 25.6 | 22.4 | 26 | 94.9 | |
| 50 | 50 | 33.0 | 27.3 | 7.7 | 42.0 | 75.0 | 93.6 | 75.4 | 99.0 |
| Hydrogel Type | C0 | QL | HT | qTh | kTh × 103 | SD |
|---|---|---|---|---|---|---|
| Dimensionless | ||||||
| Alg-Ch | 50 | 50 | 13 | 99.6 | 3.30 | 0.018 |
| 50 | 100 | 13 | 96.5 | 4.07 | 0.042 | |
| 100 | 100 | 13 | 81.1 | 5.67 | 0.033 | |
| 50 | 50 | 33 | 101.5 | 2.94 | 0.040 | |
| Alg-ChS | 50 | 50 | 13 | 86.5 | 4.50 | 0.039 |
| 50 | 100 | 13 | 79.9 | 6.21 | 0.044 | |
| 100 | 100 | 13 | 86.0 | 6.20 | 0.036 | |
| 50 | 50 | 33 | 97.3 | 2.66 | 0.045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduzco-Navarro, I.P.; Rios-Donato, N.; Jasso-Gastinel, C.F.; Martínez-Gómez, Á.d.J.; Mendizábal, E. Removal of Cu(II) by Fixed-Bed Columns Using Alg-Ch and Alg-ChS Hydrogel Beads: Effect of Operating Conditions on the Mass Transfer Zone. Polymers 2020, 12, 2345. https://doi.org/10.3390/polym12102345
Verduzco-Navarro IP, Rios-Donato N, Jasso-Gastinel CF, Martínez-Gómez ÁdJ, Mendizábal E. Removal of Cu(II) by Fixed-Bed Columns Using Alg-Ch and Alg-ChS Hydrogel Beads: Effect of Operating Conditions on the Mass Transfer Zone. Polymers. 2020; 12(10):2345. https://doi.org/10.3390/polym12102345
Chicago/Turabian StyleVerduzco-Navarro, Ilse Paulina, Nely Rios-Donato, Carlos Federico Jasso-Gastinel, Álvaro de Jesús Martínez-Gómez, and Eduardo Mendizábal. 2020. "Removal of Cu(II) by Fixed-Bed Columns Using Alg-Ch and Alg-ChS Hydrogel Beads: Effect of Operating Conditions on the Mass Transfer Zone" Polymers 12, no. 10: 2345. https://doi.org/10.3390/polym12102345