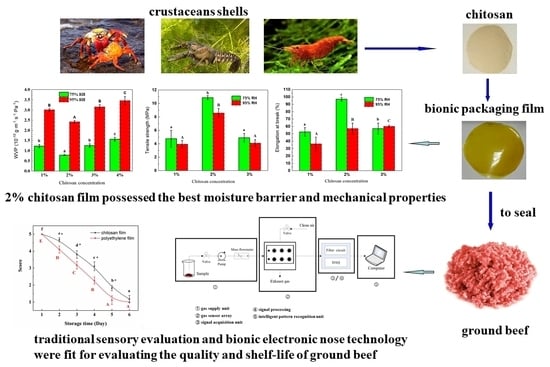
Traditional Sensory Evaluation and Bionic Electronic Nose as Innovative Tools for the Packaging Performance Evaluation of Chitosan Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GPC Film Preparation
2.3. Properties of GPC Films
2.4. Sensory Evaluation
2.5. B-Electronic Nose Detection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared (FT-IR) and X-Ray Diffraction (XRD) Analysis
3.2. Physicochemical Properties of the Chitosan Films
3.3. Water Vapor Permeability (WVP)
3.4. Mechanical Properties
3.5. Sensory Evaluation
3.6. B-Electronic Nose Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahieu, A.; Terrié, C.; Youssef, B. Thermoplastic starch films and thermoplastic starch/polycaprolactone blends with oxygen-scavenging properties: Influence of water content. Ind. Crop. Prod. 2015, 72, 192–199. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S. Active Packaging Technologies with an Emphasis on Antimicrobial Packaging and its Applications. J. Food Sci. 2003, 68, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Morphological characteristics and barrier properties of thermoplasticstarch/chitosan blown film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. A tribute to Henri Braconnot, precursor of the carbohydrate polymersscience on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar] [CrossRef] [Green Version]
- Withouck, H.; Boeykens, A.; Broucke, M.V.; Moreira, M.M.; Delerue-Matos, C.; De Cooman, L. Evaluation of the impact of pre-treatment and extraction conditions on the polyphenolic profile and antioxidant activity of Belgium apple wood. Eur. Food Res. Technol. 2019, 245, 2565–2578. [Google Scholar] [CrossRef]
- Markowicz, J.; Uram, Ł.; Sobich, J.; Mangiardi, L.; Maj, P.; Rode, W. Antitumor and anti-nematode activities of α-mangostin. Eur. J. Pharmacol. 2019, 863, 172678. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Darmadji, P.; Izumimoto, M. Effect of chitosan in meat preservation. Meat Sci. 1994, 38, 243–254. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.; Piette, J.-P.G.; Bégin, A.; Holley, R. Diffusion of Acetic and Propionic Acids from Chitosan-based Antimicrobial Packaging Films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Artharn, A.; Prodpran, T.; Benjakul, S. Round scad protein-based film: Storage stability and its effectiveness for shelf-life extension of dried fish powder. LWT Food Sci. Technol. 2009, 42, 1238–1244. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan−Whey Protein Edible Films Produced in the Absence or Presence of Transglutaminase: Analysis of Their Mechanical and Barrier Properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejska, I.; Piotrowska, B. The water vapour permeability, mechanical properties and solubility of fish gelatin–chitosan films modified with transglutaminase or 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and plasticized with glycerol. Food Chem. 2007, 103, 295–300. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- De Abreu, D.P.; Losada, P.P.; Angulo, I.; Cruz, J. Development of new polyolefin films with nanoclays for application in food packaging. Eur. Polym. J. 2007, 43, 2229–2243. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.; Krochta, J.; Mattoso, L.H.C.; Aouada, F.A. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Ren, L.; Fu, Y.; Chang, Y.; Jiang, M.; Tong, J.; Zhou, J. Performance improvement of starch films reinforced with starch nanocrystals (SNCs) modified by cross-linking. Starch Stärke 2016, 69, 1600025. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Sessini, V.; Arrieta, M.; Fernández-Torres, A.; Peponi, L. Humidity-activated shape memory effect on plasticized starch-based biomaterials. Carbohydr. Polym. 2018, 179, 93–99. [Google Scholar] [CrossRef]
- Cervera, M.F.; Karjalainen, M.; Airaksinen, S.; Rantanen, J.; Krogars, K.; Heinämäki, J.; Colarte, A.I.; Yliruusi, J. Physical stability and moisture sorption of aqueous chitosan–amylose starch films plasticized with polyols. Eur. J. Pharm. Biopharm. 2004, 58, 69–76. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.D.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Wojnowski, W.; Kalinowska, K.; Majchrzak, T.; Płotka-Wasylka, J.; Namieśnik, J. Prediction of the Biogenic Amines Index of Poultry Meat Using an Electronic Nose. Sensors 2019, 19, 1580. [Google Scholar] [CrossRef] [Green Version]
- Torri, L.; Piochi, M. Sensory methods and electronic nose as innovative tools for the evaluation of the aroma transfer properties of food plastic bags. Food Res. Int. 2016, 85, 235–243. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Ding, W.; Zhang, D.Q.; Wang, J.; Reed, K.; Zhang, B. Adulterant identification in mutton by electronic nose and gas chromatography-mass spectrometer. Food Control. 2019, 98, 431–438. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Torres, A.V.; Salgado-Delgado, R.; Bello-Pérez, L.A. Influence of the oxidation and acetylation of banana starch on the mechanical and water barrier properties of modified starch and modified starch/chitosan blend films. J. Appl. Polym. Sci. 2010, 115, 991–998. [Google Scholar] [CrossRef]
- Mayachiew, P.; Devahastin, S. Effects of drying methods and conditions on release characteristics of edible chitosan films enriched with Indian gooseberry extract. Food Chem. 2010, 118, 594–601. [Google Scholar] [CrossRef]
- Ren, L.L.; Yan, X.X.; Zhou, J.; Su, X.G. Influence of chitosan concentration on mechanical and barrierproperties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 10, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.R.; Andrade, C.T. Investigation of the gelatinization and extrusion processes of corn starch. Adv. Polym. Technol. 2002, 21, 17–24. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch–chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 035701. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Park, P.-J.; Je, J.-Y.; Kim, S.-K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Shukur, M.F.; Yusof, Y.M.; Zawawi, S.M.M.; Illias, H.A.; Kadir, M.F.Z. Conductivity and transport studies of plasticized chitosan-based proton conducting biopolymer electrolytes. Phys. Scr. 2013, 157, 014050. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K.W. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhao, L.; Yoshii, F.; Kume, T. Study on antibacterial starch/chitosan blend film formed under the action of irradiation. Carbohydr. Polym. 2004, 57, 83–88. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of sodium caseinate on properties and ageing behaviour of corn starch based films. Food Hydrocoll. 2012, 29, 265–271. [Google Scholar] [CrossRef]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical and barrier properties investigation of chitosan–clay nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Bof, M.J.; Bordagaray, V.C.; Locaso, D.E.; García, M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar] [CrossRef]
- García, M.A.; Pinotti, A.; Zaritzky, N. Physicochemical, Water Vapor Barrier and Mechanical Properties of Corn Starch and Chitosan Composite Films. Starch Stärke 2006, 58, 453–463. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.; Yamashita, F.; Pineda, E.A. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosanfilms incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Y.; Zhao, Y. Physicochemical, Microstructural, and Antibacterial Properties of ??Chitosan and Kudzu Starch Composite Films. J. Food Sci. 2012, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.; Graciano-Verdugo, A.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Chen, J.; Gu, J.; Zhang, R.; Mao, Y.; Tian, S. Freshness Evaluation of Three Kinds of Meats Based on the Electronic Nose. Sensors 2019, 19, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Wu, Y.; Guo, Q.; Ren, L.; Zhu, P.; Xu, L.; Chang, G.; Chen, G. Establishment of a Freshness-Evaluating Standard for Chilled Yellow Chicken Meat. Food Anal. Methods 2017, 10, 2629–2635. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Xu, B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules 2018, 23, 3286. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Sun, Y.; Zhang, Y.; Gao, Y.; Weng, X.; Chen, D.; David, L.; Xie, J. Bionic Optimization Design of Electronic Nose Chamber for Oil and Gas Detection. J. Bionic Eng. 2018, 15, 533–544. [Google Scholar] [CrossRef]













| Sensor Number | Series Name | Sensitive Substances |
|---|---|---|
| S1 | TGS2602 | VOC, ammonia, hydrogen sulfide |
| S2 | TGS2611 | methane |
| S3 | TGS2442 | carbon monoxide |
| S4 | TGS4161 | carbon dioxide |
| S5 | TGS2620 | alcohol, other organic solvent |
| S6 | TGS2610 | propane, butane, liquefied petroleum |
| Chitosan Concentration | Density (g cm−3) | SD (%) | WS (%) | Color | ||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | C* | ||||
| 1 % | 0.87 ± 0.01a | 85.93 ± 6.72b | 30.04 ± 0.10a | 89.61 ± 0.34a | −1.73 ± 0.06a | 14.73 ± 1.28a | 14.91 ± 1.26a | 4.99 ± 0.34a |
| 2 % | 0.96 ± 0.01b | 92.76 ± 7.13c | 32.57 ± 0.16b | 86.93 ± 0.22a | −2.90 ± 0.04b | 20.93 ± 0.90b | 21.72 ± 0.91b | 7.86 ± 0.22b |
| 3 % | 1.24 ± 0.02d | 146.71 ± 9.25d | 32.91 ± 0.12b | 67.82 ± 0.38b | 15.66 ± 0.51c | 79.11 ± 0.39d | 84.48 ± 0.48c | 31.40 ± 0.51c |
| 4 % | 1.16 ± 0.01c | 79.94 ± 5.69a | 33.01 ± 0.11c | 50.88 ± 3.30c | 33.33 ± 2.36d | 64.58 ± 7.41c | 84.79 ± 5.61c | 55.44 ± 4.04d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Xu, J.; Ren, L.; Guo, L.; Tong, J.; Wang, L.; Chang, Z. Traditional Sensory Evaluation and Bionic Electronic Nose as Innovative Tools for the Packaging Performance Evaluation of Chitosan Film. Polymers 2020, 12, 2310. https://doi.org/10.3390/polym12102310
Song W, Xu J, Ren L, Guo L, Tong J, Wang L, Chang Z. Traditional Sensory Evaluation and Bionic Electronic Nose as Innovative Tools for the Packaging Performance Evaluation of Chitosan Film. Polymers. 2020; 12(10):2310. https://doi.org/10.3390/polym12102310
Chicago/Turabian StyleSong, Wei, Jian Xu, Lili Ren, Li Guo, Jin Tong, Liyan Wang, and Zhiyong Chang. 2020. "Traditional Sensory Evaluation and Bionic Electronic Nose as Innovative Tools for the Packaging Performance Evaluation of Chitosan Film" Polymers 12, no. 10: 2310. https://doi.org/10.3390/polym12102310