Tuning the Density of Zwitterionic Polymer Brushes on PET Fabrics by Aminolysis: Effect on Antifouling Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prefunctionalization of PET Fabrics by Aminolysis Reaction
2.3. Immobilization of Initiator
2.4. ATRP Polymerization
2.5. Characterization
2.6. Determination of Functional Groups
2.7. Determination of Protein Adsorption
3. Results and Discussion
3.1. PET Prefunctionalization by Aminolysis
3.2. Synthesis of SBMA Polymer Brushes on PET
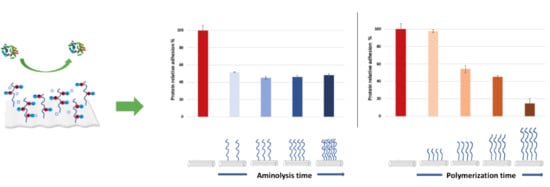
3.3. Protein Adhesion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial bofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Ahmed, S.; Darouiche, R.O. Anti-biofilm agents in control of device-related infections. Adv. Exp. Med. Biol. 2015, 831, 137–146. [Google Scholar]
- Beloin, C.; Renard, S.; Ghigo, J.-M.; Lebeaux, D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharm. 2014, 18, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Sett, S.; Yan, X.; Barac, G.; Bolton, L.; Miljkovic, N. Lubricant-Infused Surfaces for Low Surface Tension Fluids: Promise vs Reality. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y. Improvement of lubricant-infused surfaces for anti-icing applications. Surf. Innov. 2016, 4, 214–217. [Google Scholar] [CrossRef]
- Gas, A.; Keiser, L.; Clanet, C.; Quéré, D. Drop friction on liquid-infused materials. Soft Matter 2017, 13, 6981–6987. [Google Scholar]
- Manna, U.; Raman, N.; Welsh, M.A.; Zayas-Gonzalez, Y.M.; Blackwell, H.E.; Palecek, S.P.; Lynn, D.M. Slippery Liquid-Infused Porous Surfaces that Prevent Microbial Surface Fouling and Kill Non-Adherent Pathogens in Surrounding Media: A Controlled Release Approach. Adv. Funct. Mater. 2016, 26, 3599–3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Lee, J.; Andrade, J.; De Gennes, P. Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Jeon, S.; Andrade, J. Protein—surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J. Colloid Interface Sci. 1991, 142, 159–166. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Adv. Mater. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Ostaci, R.-V.; Damiron, D.; Al Akhrass, S.; Grohens, Y.; Drockenmuller, E. Poly(ethylene glycol) brushes grafted to silicon substrates by click chemistry: Influence of PEG chain length, concentration in the grafting solution and reaction time. Polym. Chem. 2011, 2, 348–354. [Google Scholar] [CrossRef]
- Favel, B.S.; Jasieniak, M.; Velleman, L.; Ciampi, S.; Luais, E.; Peterson, J.R.; Griesser, H.J.; Shapter, J.G.; Gooding, J.J. Grafting of Poly(ethylene glycol) on Click Chemistry Modified Si(100) Surfaces. Langmuir 2013, 29, 8355–8362. [Google Scholar] [CrossRef]
- Jo, S.; Park, K. Surface modification using silanated poly(ethylene glycol)s. Biomaterials 2000, 21, 605–616. [Google Scholar] [CrossRef]
- Tugulu, S.; Klok, H.-A. Stability and nonfouling properties of poly(poly(ethylene glycol) methacrylate) brushes under cell culture conditions. Biomacromolecules 2008, 9, 906–912. [Google Scholar] [CrossRef]
- Nath, N.; Hyun, J.; Ma, H.; Chilkoti, A. Surface engineering strategies for control of protein and cell interactions. Surf. Sci. 2004, 570, 98–110. [Google Scholar] [CrossRef]
- Stadler, V.; Kirmse, R.; Beyer, M.; Breitling, F.; Ludwig, T.; Bischoff, F.R. PEGMA/MMA Copolymer Graftings: Generation, Protein Resistance, and a Hydrophobic Domain. Langmuir 2008, 24, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hyun, J.; Stiller, P.; Chilkoti, A. “Non-Fouling” Oligo(ethylene glycol)- Functionalized Polymer Brushes Synthesized by Surface-Initiated Atom Transfer Radical Polymerization. Adv. Mater. 2004, 16, 338–341. [Google Scholar] [CrossRef]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Owens, D., III; Peppas, N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Metcalf, C.S.; Klein, B.D.; McDougle, D.R.; Zhang, L.; Smith, M.D.; Bulaj, G.; White, H.S. Analgesic properties of a peripherally acting and GalR2 receptor-preferring galanin analog in inflammatory, neuropathic, and acute pain models. J. Pharm. Exp. 2015, 352, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zhang, P.; Tang, X.-Y.; Zhang, C.; Ding, S.; Ye, H.; Ding, Q.-L.; Shen, W.-B.; Ping, Q.-N. PEG prodrug of gambogic acid: Amino acid and dipeptide spacer effects. Polymer (Guildf) 2012, 53, 1694–1702. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef]
- Kamphuis, M.M.J.; Johnston, A.P.R.; Such, G.K.; Dam, H.H.; Evans, R.A.; Scott, A.M.; Nice, E.C.; Heath, J.K.; Caruso, F. Targeting of cancer cells using click-functionalized polymer capsules. J. Am. Chem. Soc. 2010, 132, 15881–15883. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Such, G.K.; Johnston, A.P.R.; Zhu, Z.; Ejima, H.; Richardson, J.J.; Cui, J.; Caruso, F. Endocytic pH-Triggered Degradation of Nanoengineered Multilayer Capsules. Adv. Mater. 2013, 26, 1901–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.; Morton, S.W.; Hammond, P.T.; Swager, T.M. Fluorescent multiblock π-conjugated polymer nanoparticles for in vivo tumor targeting. Adv. Mater. 2013, 25, 4504–4510. [Google Scholar] [CrossRef] [Green Version]
- Connal, L.A.; Kinnane, C.R.; Zelikin, A.N.; Caruso, F. Stabilization and Functionalization of Polymer Multilayers and Capsules via Thiol−Ene Click Chemistry. Chem. Mater. 2009, 21, 576–578. [Google Scholar] [CrossRef]
- Leung, M.K.M.; Hagemeyer, C.E.; Johnston, A.P.R.; Gonzales, C.; Kamphuis, M.M.J.; Ardipradja, K.; Such, G.K.; Peter, K.; Caruso, F. Bio-click chemistry: Enzymatic functionalization of PEGylated capsules for targeting applications. Angew. Chem. Int. Ed. Engl. 2012, 51, 7132–7136. [Google Scholar] [CrossRef] [Green Version]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chou, Y.-N.; Venault, A.; Cho, C.-H.; Sin, M.-C.; Yeh, L.-C.; Jhong, J.-F.; Chinnathambi, A.; Chang, Y.; Chang, Y. Epoxylated Zwitterionic Triblock Copolymers Grafted onto Metallic Surfaces for General Biofouling Mitigation. Langmuir 2017, 33, 9822–9835. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, S. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar] [CrossRef]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer (Guildf) 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yuan, J. Design of hemocompatible and antifouling PET sheets with synergistic zwitterionic surfaces. J. Colloid Interface Sci. 2016, 480, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Tang, Q.; Li, L.; Humble, J.; Wu, H.; Liu, L.; Cheng, G. Switchable antimicrobial and antifouling hydrogels with enhanced mechanical properties. Adv. Healthc. Mater. 2013, 2, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Müller, A.H.E.; Matyjaszewski, K.; Sheiko, S.S. Molecular Brushes. In Polymer Science: A Comprehensive Reference, 10 Volume Set; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 199–264. ISBN 9780080878621. [Google Scholar]
- Renner, L.D.; Weibel, D.B. Physiochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Guo, S.; Jańczewski, D.; Zhu, X.; Quintana, R.; He, T.; Neoh, K.G. Surface charge control for zwitterionic polymer brushes: Tailoring surface properties to antifouling applications. J. Colloid Interface Sci. 2015, 452, 43–53. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Opwis, K.; Gutmann, J.S. Polyvinylamine modified polyester fibers—Innovative textiles for the removal of chromate from contaminated groundwater. J. Mater. Chem. A 2014, 3, 386–394. [Google Scholar] [CrossRef]
- Mehmood, T.; Kaynak, A.; Dai, X.J.; Kouzani, A.; Magniez, K.; Rubin de Celis, D.; Hurren, C.J.; du Plessis, J. Study of oxygen plasma pre-treatment of polyester fabric for improved polypyrrole adhesion. Mater. Chem. Phys. 2014, 143, 668–675. [Google Scholar] [CrossRef]
- Leroux, F.; Perwuelz, A.; Campagne, C.; Behary, N. Atmospheric air-plasma treatments of polyester textile structures. J. Adhes. Sci. Technol. 2006, 20, 939–957. [Google Scholar] [CrossRef]
- Timma, L.M.; Lewald, L.; Gier, F.; Homey, L.; Neyer, C.; Nickisch-Hartfiel, A.; Gutmann, S.; Oberthür, M. Nonofouling textiles with tunable antimicrobial activity based on a zwitterionic polyamine finish. RSC Adv. 2019, 9, 9783–9791. [Google Scholar] [CrossRef] [Green Version]
- Pelton, R. Polyvinylamine: A tool for engineering interfaces. Langmuir 2014, 30, 15373–15382. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of primary amine groups available for subsequent biofunctionalization of polymer surfaces. Bioconjug. Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, S.; Liberelle, B.; Yogi, A.; Moreno, M.J.; Bureau, M.N.; Robitaille, L.; De Crescenzo, G. A non-damaging chemical amination protocol for poly(ethylene terephthalate)-application to the design of functionalized compliant vascular grafts. J. Mater. Chem. B 2013, 1, 230–238. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Costa, L.; Pardoue, S.; Dragoé, D.; Mazerat, S.; Roger, P. Hydrophilic PET surfaces by aminolysis and glycopolymer brushes chemistry. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2689–2697. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Zhong, L.; Zhang, F.; Zhang, G. Aminolysis of polyethylene terephthalate fabric by a method involving the gradual concentration of dilute ethylenediamine. Colloids Surf. A Phys. Eng. Asp. 2017, 513, 146–152. [Google Scholar] [CrossRef]
- More, A.P.; Kokate, S.R.; Rane, P.C.; Mhaske, S.T. Studies of different techniques of aminolysis of poly(ethylene terephthalate) with ethylenediamine. Polym. Bull. 2017, 74, 3269–3282. [Google Scholar] [CrossRef]
- Hoang, C.N.; Dang, Y.H. Aminolysis of poly(ethylene terephthalate) waste with ethylenediamine and characterization of a,u-diamine products. Polym. Degrad. Stab. 2013, 98, 697–708. [Google Scholar] [CrossRef]
- Lorusso, E.; Ali, W.; Hildebrandt, M.; Mayer-Gall, T.; Gutmann, J.S. Hydrogel Functionalized Polyester Fabrics by UV-Induced Photopolymerization. Polymer (Basel) 2019, 11, 1329. [Google Scholar] [CrossRef] [Green Version]
- Ellison, M.S.; Fisher, L.D.; Alger, K.W.; Zeronian, S.H. Physical properties of polyester fibers degraded by aminolysis and by alkalin hydrolysis. J. Appl. Polym. Sci. 1982, 27, 247–257. [Google Scholar] [CrossRef]
- Muthuvijayan, V.; Gu, J.; Lewis, R.S. Analysis of functionalized polyethylene terephthalate with immobilized NTPDase and cysteine. Acta Biomater. 2009, 5, 3382–3393. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Terayama, Y.; Yamaguchi, H.; Terada, M.; Murakami, D.; Ishihara, K.; Takahara, A. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir 2012, 28, 7212–7222. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, E.; Ali, W.; Leniart, M.; Gebert, B.; Oberthür, M.; Gutmann, J.S. Tuning the Density of Zwitterionic Polymer Brushes on PET Fabrics by Aminolysis: Effect on Antifouling Performances. Polymers 2020, 12, 6. https://doi.org/10.3390/polym12010006
Lorusso E, Ali W, Leniart M, Gebert B, Oberthür M, Gutmann JS. Tuning the Density of Zwitterionic Polymer Brushes on PET Fabrics by Aminolysis: Effect on Antifouling Performances. Polymers. 2020; 12(1):6. https://doi.org/10.3390/polym12010006
Chicago/Turabian StyleLorusso, Emanuela, Wael Ali, Michael Leniart, Beate Gebert, Markus Oberthür, and Jochen S. Gutmann. 2020. "Tuning the Density of Zwitterionic Polymer Brushes on PET Fabrics by Aminolysis: Effect on Antifouling Performances" Polymers 12, no. 1: 6. https://doi.org/10.3390/polym12010006