Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Speed Melt Spinning
2.3. Characterization
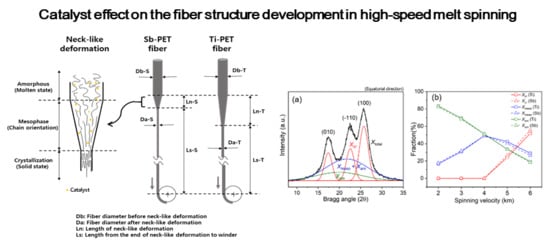
3. Results and Discussion
3.1. PET Resins
3.2. PET Fiber in High-Speed Melt Spinning
sinΦ = cosθ × sinXE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawakami, D.; Hsiao, B.S.; Ran, S.; Burger, C.; Fu, B.; Sics, I.; Chu, B.; Kikutani, T. Structural formation of amorphous poly (ethylene terephthalate) during uniaxial deformation above glass temperature. Polymer 2004, 45, 905–918. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, C.H.; Kim, S.H. Effects of pretreatment reagents on the hydrolysis and physical properties of PET fabrics. J. Appl. Polym. Sci. 2009, 112, 3071–3078. [Google Scholar] [CrossRef]
- Lee, S.J.; Hahm, W.G.; Kikutani, T.; Kim, B.C. Effects of clay and POSS nanoparticles on the quiescent and shear-induced crystallization behavior of high molecular weight poly (ethylene terephthalate). Polym. Eng. Sci. 2009, 49, 317–323. [Google Scholar] [CrossRef]
- Chen, J.W.; Chen, L.W. The kinetics of diethylene glycol formation from bis-hydroxyethyl terephthalate with antimony catalyst in the preparation of PET. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1797–1803. [Google Scholar] [CrossRef]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Martinez, C.A.C.; Reyes, L.H.; Ramirez, A.H.; Ruiz, E.R.; Trevino, L.M.; Mar, J.L.G. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Martl, M.; Mezger, T.; Kuhn, B.; Oberlein, G.; Haferland, K.; Boehringer, B.; Berger, U. Process for the Preparation of Polyesters and Copolyesters. U.S. Patent 5,789,528, 4 August 1998. [Google Scholar]
- Cannon, K.C.; Seshadri, S.R.; Dirkx, R.R. Preparation of Polyesters Using Lithium Titanyl Oxalate Polycondensation Catalysts. E. P. Patent 0,970,983A2, 7 July 2000. [Google Scholar]
- Schiraldi, D.A. Process for Producing Polyethylene Terephthalate Using a Specific Catalyst Stabilizer System. U.S. Patent 5,922,828, 13 June 1999. [Google Scholar]
- Putzig, D.E. Esterification Catalysts and Processes Therefor and Therewith. U.S. Patent 6,166,170, 26 December 2000. [Google Scholar]
- Putzig, D.E. Titanium-Containing Catalyst Composition and Processes Therefor and Therewith. U.S. Patent 6,080,834, 27 June 2000. [Google Scholar]
- Lustig, S.R.; Burch, R.R.; McCarron, E.M. Catalysis with Titanium Oxides. U.S. Patent 6,034,203, 7 March 2000. [Google Scholar]
- MacDonald, W.A. New advances in poly (ethylene terephthalate) polymerization and degradation. Polym. Int. 2002, 51, 923–930. [Google Scholar] [CrossRef]
- Thier-Grebe, R.; Rabe, M. Polyester with titanium dioxide, catalyst ‘C-94’. Prop. Acordis 2000, 119–128. Available online: https://www.tib.eu/en/search/id/BLSE%3ARN073847072/Polyester-With-New-Titanium-Dioxide-Catalyst-C/ (accessed on 21 November 2019).
- Thiele, U.K. The current status of catalysis and catalyst development for the industrial process of poly (ethylene terephthalate) polycondensation. Int. J. Polym. Mater. 2001, 50, 387–394. [Google Scholar] [CrossRef]
- Finelli, L.; Lorenzetti, C.; Messori, M.; Sisti, L.; Vannini, M. Comparison between titanium tetrabutoxide and a new commercial titanium dioxide based catalyst used for the synthesis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2004, 92, 1887–1892. [Google Scholar] [CrossRef]
- Traub, H.L.; Hirt, P.; Herlinger, H.; Oppermann, W. Synthesis and properties of fiber-grade poly (trimethylene terephthalate). Angew. Makromol. Chem. 1995, 230, 179–187. [Google Scholar] [CrossRef]
- Duh, B. Effect of antimony catalyst on solid-state polycondensation of poly (ethylene terephthalate). Polymer 2002, 43, 3147–3154. [Google Scholar] [CrossRef]
- Shimizu, J.; Kikutani, T. Dynamics and evolution of structure in fiber extrusion. J. Appl. Polym. Sci. 2002, 83, 539–558. [Google Scholar] [CrossRef]
- Cao, J. Spinning the supercooled PET to obtain highly oriented and crystallized PET fibers at low speeds. J. Appl. Polym. Sci. 2006, 102, 3078–3082. [Google Scholar] [CrossRef]
- Alexander, L.E. X-ray Diffraction Methods in Polymer Science, 1st ed.; Wiley-Interscience, Cop.: New York, NY, USA, 1969. [Google Scholar]
- Pilati, F.; Toselli, M.; Messori, M.; Manzoni, C.; Turturro, A.; Gattiglia, E.G. On specific factors affecting the crystallization of PET: The role of carboxyl terminal groups and residual catalysts on the crystallization rate. Polymer 1997, 38, 4469–4476. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Wu, Y.; Cuculo, J.A. Structure and property studies of poly (trimethylene terephthalate) high-speed melt spun fibers. Polymer 2002, 43, 4915–4922. [Google Scholar] [CrossRef]
- Lu, Y.H.; Lin, H.; Chen, Y.Y.; Wang, C.; Hua, Y.R. Structure and performance of Bombyx mori silk modified with nano-TiO2 and chitosan. Fibers Polym. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Ziabicki, A.; Jarecki, L.; Wasiak, A. Dynamic modelling of melt spinning. Comput. Theor. Polym. Sci. 1998, 8, 143–157. [Google Scholar] [CrossRef]
- Hahm, W.G. Mechanism of Fiber Structure Development in High-Speed In-Line Drawing Process of Poly (ethylene Terephthalate). Ph.D. Thesis, Tokyo Institute of Technology, Tokyo, Japan, September 2006. [Google Scholar]
- Kikutani, T. Structure of Polymer materials, 1st ed.; Kyoritsu Shuppan: Tokyo, Japan, 1997; pp. 481–507. [Google Scholar]








| Spinning Velocity (km/min) | Catalyst | |
|---|---|---|
| TiO2 | Sb2O3 | |
| 2 | Ti-PET-2 | Sb-PET-2 |
| 3 | Ti-PET-3 | Sb-PET-3 |
| 4 | Ti-PET-4 | Sb-PET-4 |
| 5 | Ti-PET-5 | Sb-PET-5 |
| 6 | Ti-PET-6 | Sb-PET-6 |
| Sample | IV (dl/g) | GPC | ICP | TGA 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Mn | Mw | Mw/Mn | Sb (ppm) | 99% | 90% | |||
| Ti-PET | Resin | 0.634 (0.602 2) | 4153 | 11,235 | 2.70 | - | 369 | 398 |
| Sb-PET | Resin | 0.630 (0.592 2) | 4124 | 10,986 | 2.66 | 244 (163 2) | 372 | 402 |
| Sample | Temperature (°C) | t1/2 (s) | n | K (s−1) | ||
|---|---|---|---|---|---|---|
| n1 | n2 | K1 | K2 | |||
| Ti-PET | 195 | 239 | 1.1 | 2.7 | 27.5 × 10−6 | 2.2 × 10−7 |
| 205 | 471 | 1.4 | 2.7 | 4.0 × 10−6 | 0.5 × 10−7 | |
| Sb-PET | 195 | 139 | - | 2.4 | - | 43.0 × 10−7 |
| 205 | 241 | 1.3 | 2.5 | 25.1 × 10−6 | 7.9 × 10−7 | |
| Sample | βmeso 1 | Mass Fraction 2 (%) | dspacing (Å) | Crystallite Size 3 (Å) | No. of Unit Cells 4 | fc5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (deg) | Xcr | Xmeso | Xam | (010) | (100) | (−103) | (010) | (100) | (−103) | (010) | (100) | (−103) | (010) | |
| Ti-PET-2 | 39.4 | 0.0 | 16.6 | 83.4 | - | - | - | - | - | - | - | - | - | - |
| Ti-PET-3 | 33.6 | 0.0 | 30.7 | 69.3 | - | - | - | - | - | - | - | - | - | - |
| Ti-PET-4 | 26.7 | 0.0 | 49.1 | 50.9 | - | - | - | - | - | - | - | - | - | - |
| Ti-PET-5 | 24.8 | 24.2 | 42.3 | 33.5 | 5.22 | 3.54 | 3.44 | 31.3 | 24.1 | 39.6 | 6.0 | 6.8 | 11.5 | 0.893 |
| Ti-PET-6 | 20.1 | 51.7 | 28.9 | 19.4 | 5.08 | 3.45 | 3.40 | 40.6 | 34.9 | 54.7 | 8.0 | 10.1 | 16.1 | 0.921 |
| Sb-PET-2 | 38.1 | 0.0 | 17.7 | 82.3 | - | - | - | - | - | - | - | - | - | - |
| Sb-PET-3 | 35.2 | 0.0 | 31.8 | 68.2 | - | - | - | - | - | - | - | - | - | - |
| Sb-PET-4 | 26.5 | 0.0 | 48.9 | 51.1 | - | - | - | - | - | - | - | - | - | - |
| Sb-PET-5 | 22.1 | 26.6 | 40.0 | 33.5 | 5.21 | 3.51 | 3.46 | 30.8 | 26.1 | 44.5 | 5.9 | 7.4 | 12.9 | 0.897 |
| Sb-PET-6 | 18.8 | 55.4 | 26.4 | 18.2 | 5.09 | 3.46 | 3.41 | 45.7 | 37.7 | 55.9 | 9.0 | 10.9 | 16.4 | 0.925 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.S.; Oh, H.J.; Kim, H.-J.; Kim, C.G.; Park, S.Y.; Jeong, Y.G.; Hahm, W.-G. Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate). Polymers 2019, 11, 1931. https://doi.org/10.3390/polym11121931
Kim ES, Oh HJ, Kim H-J, Kim CG, Park SY, Jeong YG, Hahm W-G. Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate). Polymers. 2019; 11(12):1931. https://doi.org/10.3390/polym11121931
Chicago/Turabian StyleKim, Eun Seon, Hyun Ju Oh, Hyun-Joong Kim, Chun Gi Kim, Seong Yoon Park, Young Gyu Jeong, and Wan-Gyu Hahm. 2019. "Effect of Polycondensation Catalyst on Fiber Structure Development in High-Speed Melt Spinning of Poly (Ethylene Terephthalate)" Polymers 11, no. 12: 1931. https://doi.org/10.3390/polym11121931