Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide
Abstract
:1. Introduction
2. Experimental
2.1. Materials
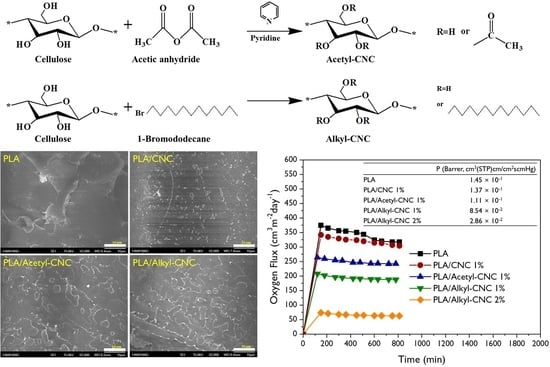
2.2. Surface Modifications of CNCs
2.3. Determination of the Degree of Substitution by Titration
2.4. Fabrication of PLA/Modified CNC Composite Films
2.5. Characterization
3. Results and Discussion
3.1. Surface Modifications of CNCs
3.1.1. Chemical Structures of CNC and Modified CNCs
3.1.2. Morphology of Modified and Unmodified CNCs
3.1.3. Contact Angle Analysis and Surface Free Energy of CNCs and Modified CNCs
3.2. Properties of PLA Nanocomposites
3.2.1. Fracture Morphology
3.2.2. Crystallinity
3.2.3. Thermal Stability
3.2.4. Mechanical Properties and Modeling
3.2.5. Barrier Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Oh, K.W.; Kim, S.H. Reinforcement effect of cellulose nanowhisker on bio-based polyurethane. Compos. Sci. Technol. 2013, 86, 82–88. [Google Scholar] [CrossRef]
- Rahman, A.M.; Alimuzzaman, S.; Khan, R.A.; Hossen, J. Evaluating the performance of gamma irradiated okra fiber reinforced polypropylene (PP) composites: Comparative study with jute/PP. Fash. Text. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Wang, D.; Yu, J.; Zhang, J.; He, J.; Zhang, J. Transparent bionanocomposites with improved properties from poly (propylene carbonate) (PPC) and cellulose nanowhiskers (CNWs). Compos. Sci. Technol. 2013, 85, 83–89. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Trittschuh, S.; Seidel, S.; Rowan, S.J.; Weder, C. Polymer nanocomposites with nanowhiskers isolated from microcrystalline cellulose. Biomacromolecules 2009, 10, 712–716. [Google Scholar] [CrossRef]
- Cunha, A.G.; Zhou, Q.; Larsson, P.T.; Berglund, L.A. Topochemical acetylation of cellulose nanopaper structures for biocomposites: Mechanisms for reduced water vapour sorption. Cellulose 2014, 21, 2773–2787. [Google Scholar] [CrossRef] [Green Version]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A.; Gandini, A. Modification of cellulose fibers with functionalized silanes: Effect of the fiber treatment on the mechanical performances of cellulose–thermoset composites. J. Appl. Polym. Sci. 2005, 98, 974–984. [Google Scholar] [CrossRef]
- Ten, E.; Turtle, J.; Bahr, D.; Jiang, L.; Wolcott, M. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Polymer 2010, 51, 2652–2660. [Google Scholar] [CrossRef]
- Pei, A.; Zhou, Q.; Berglund, L.A. Functionalized cellulose nanocrystals as biobased nucleation agents in poly (l-lactide) (PLLA)–Crystallization and mechanical property effects. Compos. Sci. Technol. 2010, 70, 815–821. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and thermal properties of poly (lactic acid)/cellulose whiskers nanocomposite materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of cellulose nanocrystal and its reinforcing function in poly (lactic acid). Carbohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Kanno, T.; Asoh, T.-A.; Uyama, H. Surface modification of cellulose nanofiber using acid anhydride for poly (lactic acid) reinforcement. Mater. Today Commun. 2019, 21, 100587. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly (ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Kloser, E.; Gray, D.G. Surface grafting of cellulose nanocrystals with poly (ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef]
- Habibi, Y.; Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Wang, X.; Gao, Q.; Li, X. Surface modification of cellulose nanocrystal using succinic anhydride and its effects on poly (butylene succinate) based composites. Cellulose 2019, 26, 3167–3181. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient removal of UO22+ from water using carboxycellulose nanofibers prepared by the nitro-oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, M.N.; Gandini, A. The surface modification of cellulose fibres for use as reinforcing elements in composite materials. Compos. Interfaces 2005, 12, 41–75. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.G.; Kim, S.H. Isothermal crystallization behavior and mechanical properties of polylactide/carbon nanotube nanocomposites. Compos. Part. A-Appl. Sci. Manuf. 2013, 46, 11–18. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, Y.S.; Kim, I.S.; Kim, S.H. Pretreatment of Microfibrillated Cellulose on Polylactide Composites. Macromol. Res. 2019. [Google Scholar] [CrossRef]
- Lee, J.S.; Ryu, Y.S.; Kim, I.S.; Kim, S.H. Effect of interface affinity on the performance of a composite of microcrystalline cellulose and polypropylene/polylactide blends. Polym. Int. 2019, 68, 1402–1410. [Google Scholar] [CrossRef]
- Mpofu, N.S.; Mwasiagi, J.I.; Nkiwane, L.C.; Njuguna, D. Use of regression to study the effect of fabric parameters on the adhesion of 3D printed PLA polymer onto woven fabrics. Fash. Text. 2019, 6, 24. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly (lactic) acid films: Effect of cellulose nanocrystals and a masterbatch process. RSC Adv. 2015, 5, 32350–32357. [Google Scholar] [CrossRef]
- Martino, V.P.; Ruseckaite, R.A.; Jiménez, A.; Averous, L. Correlation between Composition, Structure and Properties of Poly (lactic acid)/Polyadipate-Based Nano-Biocomposites. Macromol. Mater. Eng. 2010, 295, 551–558. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile method to functionalize graphene oxide and its application to poly (ethylene terephthalate)/graphene composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Philpp, B.; Heinze, T.; Hewinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry. Volume 2: Functionalization of Cellulose; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998. [Google Scholar]
- Lee, J.H.; Park, S.H.; Kim, S.H. Surface modification of cellulose nanowhiskers and their reinforcing effect in polylactide. Macromol. Res. 2014, 22, 424–430. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, S.H. Alkylation of mixed micro-and nanocellulose to improve dispersion in polylactide. Polym. Int. 2015, 64, 821–827. [Google Scholar] [CrossRef]
- Atalla, R.H.; Gast, J.; Sindorf, D.; Bartuska, V.; Maciel, G. Carbon-13 NMR spectra of cellulose polymorphs. J. Am. Chem. Soc. 1980, 102, 3249–3251. [Google Scholar] [CrossRef]
- Park, S.H.; Ryu, Y.S.; Kim, S.H. Effect of modified silica nanoparticle on the properties of bio-based polyurethane ultrafine fibers. J. Mater. Sci. 2015, 50, 1760–1769. [Google Scholar] [CrossRef]
- Zini, E.; Scandola, M.; Gatenholm, P. Heterogeneous acylation of flax fibers. Reaction kinetics and surface properties. Biomacromolecules 2003, 4, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Sava, I.; Chisca, S.; Wolinska-Grabczyk, A.; Jankowski, A.; Sava, M.; Grabiec, E.; Bruma, M. Synthesis and thermal, mechanical and gas permeation properties of aromatic polyimides containing different linkage groups. Polym. Int. 2015, 64, 154–164. [Google Scholar] [CrossRef]
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lee, J.H.; Park, S.H.; Kim, S.H. Preparation of cellulose nanowhiskers and their reinforcing effect in polylactide. Macromol. Res. 2013, 21, 1218–1225. [Google Scholar] [CrossRef]
- Matsumura, H.; Sugiyama, J.; Glasser, W.G. Cellulosic nanocomposites. I. Thermally deformable cellulose hexanoates from heterogeneous reaction. J. Appl. Polym. Sci. 2000, 78, 2242–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of phase transitional behavior of poly (l-lactide)/poly (d-lactide) blend used to prepare the highly-oriented stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- Ho Park, S.; Goo Lee, S.; Kim, S.H. Thermal decomposition behavior of carbon nanotube reinforced thermotropic liquid crystalline polymers. J. Appl. Polym. Sci. 2011, 122, 2060–2070. [Google Scholar] [CrossRef]
- Spinella, S.; Re, G.L.; Liu, B.; Dorgan, J.; Habibi, Y.; Leclere, P.; Raquez, J.-M.; Dubois, P.; Gross, R.A. Polylactide/cellulose nanocrystal nanocomposites: Efficient routes for nanofiber modification and effects of nanofiber chemistry on PLA reinforcement. Polymer 2015, 65, 9–17. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Wang, C.-C.; Chen, C.-Y. Functionalizing carbon nanotubes by plasma modification for the preparation of covalent-integrated epoxy composites. Chem. Mater. 2007, 19, 308–315. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with cellulose nanocrystal/silver nanohybrids as bifunctional nanofillers. J. Mater. Chem. B. 2014, 2, 8479–8489. [Google Scholar] [CrossRef]
- Taniguchi, A.; Cakmak, M. The suppression of strain induced crystallization in PET through sub micron TiO2 particle incorporation. Polymer 2004, 45, 6647–6654. [Google Scholar] [CrossRef]
- Hull, D. An Introduction to Composite Materials; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Halpin, J.C.; Kardos, J.L. Moduli of crystalline polymers employing composite theory. J. Appl. Phys. 1972, 43, 2235. [Google Scholar] [CrossRef]
- Dalmas, F.; Cavaillé, J.-Y.; Gauthier, C.; Chazeau, L.; Dendievel, R. Viscoelastic behavior and electrical properties of flexible nanofiber filled polymer nanocomposites. Influence of processing conditions. Compos. Sci. Technol. 2007, 67, 829–839. [Google Scholar] [CrossRef]
- Malaika, S.A.; Golovoy, A.; Wilkie, C.A. Specialty Polymer Additives; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Petersson, L.A.; Oksman, K.A. Biopolymer based nanocomposites: Comparing layered silicates and microcrystalline cellulose as nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]








| Chemical Structure | Characterization | |
|---|---|---|
| FT-IR analysis |  | 3350 cm−1: O-H band 1740 cm−1: C = O stretching 1370 cm−1: C-CH3 in-plane bending 1230 cm−1: C-O stretching 2853 and 2926 cm−1: C-H stretching |
| Solid state 13C NMR analysis |  |  |
| Water | Methylene Iodide | γSd (mJ/m2) | γSp (mJ/m2) | γS (mJ/m2) | |
|---|---|---|---|---|---|
| CNC | ≤5° | 20.1 ± 0.8° | 32.2 | 41.4 | 73.6 |
| Acetyl-CNC | 78.3 ± 1.0° | 35.3 ± 0.6° | 38.1 | 4.14 | 42.2 |
| Alkyl-CNC | 84.9 ± 1.2° | 33.8 ± 0.9° | 40.8 | 1.73 | 42.5 |
| Materials | T5a (°C) | Tdm (°C) | WRb (%) | Horowitz-Metzger | |
|---|---|---|---|---|---|
| Ea (kJ) | r2 | ||||
| PLA | 310.5 | 357.3 | 0.7 | 211.7 | 0.99 |
| PLA/CNC 1% | 294.2 | 353.7 | 1.1 | 162.1 | 0.99 |
| PLA/Acetyl-CNC 1% | 328.1 | 365.8 | 1.2 | 282.6 | 0.99 |
| PLA/Alkyl-CNC 1% | 319.6 | 361.1 | 1.5 | 241.1 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Park, S.H.; Kim, S.H. Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide. Polymers 2020, 12, 178. https://doi.org/10.3390/polym12010178
Lee JH, Park SH, Kim SH. Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide. Polymers. 2020; 12(1):178. https://doi.org/10.3390/polym12010178
Chicago/Turabian StyleLee, Joo Hyung, Sang Ho Park, and Seong Hun Kim. 2020. "Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide" Polymers 12, no. 1: 178. https://doi.org/10.3390/polym12010178