A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks
Abstract
:1. Introduction
2. Materials and Methods
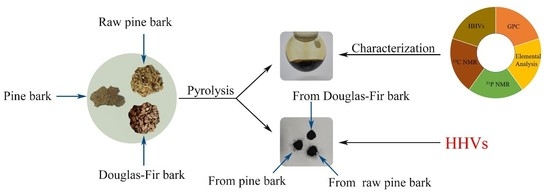
2.1. Material Preparation
2.2. Procedure of Pyrolysis Barks
2.3. Measurement of Higher Heating Value (HHV)
2.4. Measurement of Molecular Weights
2.5. Elemental Analysis of Pyrolysis Products
2.6. Structural Analysis of Pyrolysis Oil
2.6.1. Quantitative 13C NMR
2.6.2. Quantitative 31P NMR
3. Results and Discussion
3.1. Yields of Pyrolysis Products
3.2. Molecular Weights and Elemental Analysis of Pyrolysis oil
3.3. NMR Analysis of Pyrolysis Oil
3.4. Pyrolysis Mechanism Analysis
4. Industrial Application of Pyrolysis Oil
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal liquefaction for algal biorefinery: A critical review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, G.; Hu, S.; Xiang, J.; Hu, X.; Wang, Y.; Geng, D. Hydrogenation of fourteen biomass-derived phenolics in water and in methanol: Their distinct reaction behaviours. Sustain. Energy Fuels 2018, 2, 751–758. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- David, K.; Ragauskas, A.J. Switchgrass as an energy crop for biofuel production: A review of its ligno-cellulosic chemical properties. Energy Environ. Sci. 2010, 3, 1182. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J.; Tuskan, G.A. Poplar as a feedstock for biofuels: A review of compositional characteristics. Biofuels Bioprod. Biorefining 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Priyanka, R.S.; Sunil, K.S.; Richard, A.; Benjamin, S.H. Efficient removal of arsenic using zinc oxide nanocrystal-decorated regenerated microfibrillated cellulose scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar]
- Priyanka, R.S.; Aurnov, C.; Sunil, K.S.; Geng, L.H.; Nasim, A.; Darren, M.; Benjamin, S.H. Nanocellulose from spinifex as an effective adsorbent to remove cadmium(II) from water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar]
- Geng, L.H.; Peng, X.F.; Zhan, C.B.; Ali, N.; Priyanka, R.S.; Mao, Y.M.; Benjamin, S.H. Structure characterization of cellulose nanofiber hydrogel as functions of concentration and ionic strength. Cellulose 2017, 24, 5417–5429. [Google Scholar] [CrossRef]
- Priyanka, R.S.; Zheng, B.Q.; Sunil, K.S.; Zhan, C.B.; Wang, R.F.; Surita, R.B.; Benjamin, S.H. High aspect ratio carboxycellulose nanofibers prepared by nitro-oxidation method and their nanopaper properties. Appl. Nano Mater. 2018, 1, 3969–3980. [Google Scholar]
- Priyanka, R.S.; Aurnov, C.; Sunil, K.S.; Benjamin, S.H. Efficient removal of UO22+ from water using carboxycellulose nanofibers prepared by the nitro-oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar]
- Priyanka, R.S.; Ritika, J.; Sunil, K.S.; Benjamin, S.H. A simple approach to prepare carboxycellulose nanofibers from untreated biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar]
- Amen-Chen, C.; Riedl, B.; Wang, X.M.; Roy, C. Softwood Bark Pyrolysis Oil-PF Resols. Part 1. Resin Synthesis and OSB Mechanical Properties. Holzforschung 2002, 56, 167–175. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using tga–ftir and model-free integral methods. Energy Convers. Manag. 2014, 89, 251–259. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.C.; Dong, C.Q.; Zhang, Z.F.; Zhang, X.M.; Zhu, X.F. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical py-gc/ms study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Mourant, D.; Lievens, C.; Gunawan, R.; Wang, Y.; Hu, X.; Wu, L.; Syed-Hassan, S.S.A.; Li, C.-Z. Effects of temperature on the yields and properties of bio-oil from the fast pyrolysis of mallee bark. Fuel 2013, 108, 400–408. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Yassir, M.; Yehya, E.S.; Mubarak, S.; Paul, N.; Scott, B.; Tony, B. Fast pyrolysis of date palm (Phoenix dactylifera) waste in a bubbling fluidized bed reactor. Renew. Energy 2019, 143, 719–730. [Google Scholar]
- Pinto, O.; Romero, R.; Carrier, M.; Appelt, J.; Segura, C. Fast pyrolysis of tannins from pine bark as a renewable source of catechols. J. Anal. Appl. Pyrolysis 2018, 136, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.A.L.; Machado, M.E.; Caramão, E.B. Characterization of bio-oils obtained from pyrolysis of bocaiuva residues. Renew. Energy 2016, 91, 21–31. [Google Scholar] [CrossRef]
- Ranjeet, K.M.; Kaustubha, M. Pyrolysis of three waste biomass: Effect of biomass bed thickness and distance between successive beds on pyrolytic products yield and properties. Renew. Energy 2019, 141, 549–558. [Google Scholar]
- Sakthivel, R.; Ramesh, K.; Shameer, P.M.; Purnachandran, R. A Complete Analytical Characterization of Products Obtained from Pyrolysis of Wood Barks of Calophyllum inophyllum. Waste Biomass Valorization 2018, 10, 2319–2333. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, C.; Liu, H.; Wang, Y.; Guo, J. Reaction mechanism and evolved gases of larch bark pyrolysis by tg-ftir analysis. Wood Sci. Technol. 2019, 53, 101–118. [Google Scholar] [CrossRef]
- Schellekens, J.; Silva, C.A.; Buurman, P.; Rittl, T.F.; Domingues, R.R.; Justi, M.; Vidal-Torrado, P.; Trugilho, P.F. Molecular characterization of biochar from five Brazilian agricultural residues obtained at different charring temperatures. J. Anal. Appl. Pyrolysis 2018, 130, 106–117. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liang, T.; Zhou, Y.; Luo, Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012, 104, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhuo, J.; Zhang, B.; Yao, Q. Effect of moisture content on the characterization of products from the pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2013, 104, 632–639. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Pyrolysis of Kraft Lignin with Additives. Energy Fuels 2011, 25, 4662–4668. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Heteronuclear Single-Quantum Correlation–Nuclear Magnetic Resonance (HSQC–NMR) Fingerprint Analysis of Pyrolysis Oils. Energy Fuels 2011, 25, 5791–5801. [Google Scholar] [CrossRef]
- Kosa, M.; Ben, H.; Theliander, H.; Ragauskas, A.J. Pyrolysis oils from CO2 precipitated Kraft lignin. Green Chem. 2011, 13, 3196–3202. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Torrefaction of Loblolly pine. Green Chem. 2012, 14, 72–76. [Google Scholar] [CrossRef]
- Hu, Z.; Sykes, R.; Davis, M.F.; Charles Brummer, E.; Ragauskas, A.J. Chemical profiles of switchgrass. Bioresour. Technol. 2010, 101, 3253–3257. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Pu, Y.; Foston, M.; Ragauskas, A.J. Compositional characterization and pyrolysis of loblolly pine and douglas-fir bark. BioEnergy Res. 2013, 6, 24–34. [Google Scholar] [CrossRef]
- Şensöz, S.; Can, M. Pyrolysis of Pine (Pinus Brutia Ten.) Chips: 1. Effect of Pyrolysis Temperature and Heating Rate on the Product Yields. Energy Sources 2002, 24, 347–355. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q.; Zhu, X.F.; Zhang, Y. Catalytic Fast Pyrolysis of Cellulose to Prepare Levoglucosenone Using Sulfated Zirconia. Chem. Sus. Chem. 2011, 4, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Ingram, L.; Mohan, D.; Bricka, M.; Steele, P.; Strobel, D.; Crocker, D.; Mitchell, B.; Mohammad, J.; Cantrell, K.; Pittman, C.U. Pyrolysis of Wood and Bark in an Auger Reactor: Physical Properties and Chemical Analysis of the Produced Bio-oils. Energy Fuels 2008, 22, 614–625. [Google Scholar] [CrossRef]
- Ren, X.; Meng, J.; Chang, J.; Kelley, S.S.; Jameel, H.; Park, S. Effect of blending ratio of loblolly pine wood and bark on the properties of pyrolysis bio-oils. Fuel Process. Technol. 2017, 167, 43–49. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.; Donnison, I.; Yates, N.; Jones, J.; Jones, J. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Prins, M.; Ptasinski, K.; Janssen, F. From coal to biomass gasification: Comparison of thermodynamic efficiency. Energy 2007, 32, 1248–1259. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Towards a Lignincellulosic Biorefinery: Direct One-Step Conversion of Lignin to Hydrogen-Enriched Biofuel. Energy Fuels 2008, 22, 1371–1379. [Google Scholar] [CrossRef]
- David, K.; Ben, H.; Muzzy, J.; Feik, C.; Iisa, K.; Ragauskas, A. Chemical characterization and water content determination of bio-oils obtained from various biomass species using 31 P NMR spectroscopy. Biofuels 2012, 3, 123–128. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Jin, L.J.; Li, J.G.; Bao, Z.X.; Li, Y.; Hu, H.Q. Fast pyrolysis behaviors of cedar in an infrared-heated fixed-bed reactor. Bioresour. Technol. 2019, 290, 121739. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. Pyrolytic Behavior of Major Biomass Components in Waste Biomass. Polymers 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B.; Jung, H.J.G.; Lamb, J.F.S. Production of Bio-oil from Alfalfa Stems by Fluidized-Bed Fast Pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Mullen, C.A.; Strahan, G.D.; Boateng, A.A. Characterization of Various Fast-Pyrolysis Bio-Oils by NMR Spectroscopy. Energy Fuels 2009, 23, 2707–2718. [Google Scholar] [CrossRef]
- Ben, H.; Yoo, C.G.; Hao, N.; Adhikari, S.; Ragauskas, A.J. Review of NMR Characterization of Pyrolysis Oils. Energy Fuels 2016, 30, 6863–6880. [Google Scholar]
- Negahdar, L.; Gonzalez-Quiroga, A.; Otyuskaya, D.; Toraman, H.E.; Liu, L.; Jastrzebski, J.T.B.H.; Van Geem, K.M.; Marin, G.B.; Thybaut, J.W.; Weckhuysen, B.M. Characterization and Comparison of Fast Pyrolysis Bio-oils from Pinewood, Rapeseed Cake, and Wheat Straw Using 13C NMR and Comprehensive GC × GC. ACS Sustain. Chem. Eng. 2016, 4, 4974–4985. [Google Scholar] [CrossRef] [PubMed]
- Elias, V.O.; Simoneit, B.R.; Cordeiro, R.C.; Turcq, B. Evaluating levoglucosan as an indicator of biomass burning in Carajás, amazônia: A comparison to the charcoal record. Geochim. Cosmochim. Acta 2001, 65, 267–272. [Google Scholar] [CrossRef]
- Mattos, C.; Veloso, M.; Romeiro, G.; Folly, E. Biocidal applications trends of bio-oils from pyrolysis: Characterization of several conditions and biomass, a review. J. Anal. Appl. Pyrolysis 2019, 139, 1–12. [Google Scholar] [CrossRef]
- Zabelkin, S.; Grachev, A.; Fayzrakhmanova, G.; Makarov, A.; Bashkirov, V. Application of the water-insoluble pyrolysis oil fraction as an organic binder. Constr. Build. Mater. 2016, 102, 59–64. [Google Scholar] [CrossRef]



| Softwood Bark | Temperature (°C) | Pyrolysis Oil (%) | Char 1 (%) |
|---|---|---|---|
| Raw pine bark | 400 | 27.78 | 39.69 |
| 500 | 29.18 | 34.74 | |
| 600 | 28.41 | 31.05 | |
| Douglas-Fir bark | 500 | 26.65 | 37.55 |
| Pine bark | 500 | 26.67 | 36.68 |
| Softwood Bark | Temperature (°C) | HHV (MJ/kg) | Energy Densification Ratio 4 | Mass Yield (%) | Energy Yield 5 (%) |
|---|---|---|---|---|---|
| Raw pine bark 1 | 400 | 24.57 | 1.15 | 27.78 | 31.84 |
| 500 | 25.29 | 1.18 | 29.18 | 34.42 | |
| 600 | 24.54 | 1.13 | 28.41 | 32.19 | |
| Douglas-Fir 2 bark | 500 | 26.70 | 1.13 | 26.65 | 30.16 |
| Pine bark 3 | 500 | 25.67 | 1.19 | 26.67 | 31.61 |
| Softwood Bark | Temperature (°C) | HHV (MJ/kg) 1 | Energy Densification Ratio 5 | Mass Yield (%) | Energy Yield 6 (%) |
|---|---|---|---|---|---|
| Raw pine bark 2 | 400 | 29.34 | 1.37 | 39.69 | 54.31 |
| 500 | 31.61 | 1.47 | 34.74 | 51.22 | |
| 600 | 33.76 | 1.56 | 31.05 | 48.40 | |
| Douglas-Fir 3 bark | 500 | 31.11 | 1.32 | 37.55 | 49.52 |
| Pine bark 4 | 500 | 31.77 | 1.47 | 36.68 | 53.80 |
| Softwood Bark | Temperature (°C) | Mn (g/mol) | Mw (g/mol) | Mw/Mn |
|---|---|---|---|---|
| Raw pine bark | 400 | 250 | 389 | 1.56 |
| 500 | 263 | 433 | 1.65 | |
| 600 | 261 | 411 | 1.57 | |
| Douglas-Fir bark | 500 | 294 | 489 | 1.66 |
| Pine bark | 500 | 260 | 430 | 1.66 |
| Softwood Bark | Temperature (°C) | C% | H% | N% | O% | S% |
|---|---|---|---|---|---|---|
| Raw pine bark | 400 | 60.82 | 7.14 | 0.79 | 31.25 | - |
| 500 | 61.08 | 6.96 | 0.57 | 31.39 | - | |
| 600 | 61.31 | 7.18 | 0.88 | 30.63 | - | |
| Douglas-Fir bark | 500 | 67.54 | 8.15 | 1.42 | 22.62 | 0.27 |
| Pine bark | 500 | 61.46 | 7.54 | 0.50 | 29.64 | 0.86 |
| Hydroxyl Group Contents (mmol/g Pyrolysis Oil) | Aliphatic OH | C5 Substituted Guaiacyl Phenolic OH | Catechol, Guaiacol and p-Hydroxy-phenyl OH | Acid-OH |
|---|---|---|---|---|
| Integration region [27] (ppm) | 150.0–145.5 | 144.7–140.2 | 140.2–137.3 | 136.6–133.6 |
| Raw pine bark | 2.63 | 0.64 | 1.96 | 1.07 |
| Douglas-Fir bark | 1.66 | 0.64 | 2.73 | 1.47 |
| Pine bark | 3.25 | 0.80 | 2.50 | 1.13 |
| Integration Region (ppm) | Structure | Raw Pine Bark | Douglas-Fir Bark | Pine Bark | |
|---|---|---|---|---|---|
| Carbonyl | 215.0–166.5 |  | 6.53 | 3.84 | 5.89 |
| Aromatic C–O | 166.5–142.0 |  | 12.67 | 12.00 | 8.87 |
| Aromatic C–C | 142.0–125.0 |  | 7.77 | 4.67 | 7.14 |
| Aromatic C–H | 125.0–95.8 |  | 20.79 | 22.33 | 22.71 |
| Levoglucosan | C1 102.3, C2 72.0, C3 73.7, C4 71.7, C5 76.5, C6 64.9 |  | 14.33 | 5.19 | 14.32 |
| Aliphatic C–O | 95.8–60.8 |  | 21.41 | 5.98 | 15.79 |
| Methoxyl | 60.8–55.2 |  | 3.78 | 4.22 | 3.07 |
| Aliphatic C–C | 55.2–0.0 |  | 27.05 | 46.97 | 36.53 |
| Methyl-Aromatic | 21.6–19.1 |  | 5.09 | 3.60 | 5.86 |
| Methyl-Aromatic at ortho position of a hydroxyl or methoxyl group | 16.1–15.4 |  | 0.69 | 1.14 | 0.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben, H.; Wu, F.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A.J. A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks. Polymers 2019, 11, 1387. https://doi.org/10.3390/polym11091387
Ben H, Wu F, Wu Z, Han G, Jiang W, Ragauskas AJ. A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks. Polymers. 2019; 11(9):1387. https://doi.org/10.3390/polym11091387
Chicago/Turabian StyleBen, Haoxi, Fengze Wu, Zhihong Wu, Guangting Han, Wei Jiang, and Arthur J. Ragauskas. 2019. "A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks" Polymers 11, no. 9: 1387. https://doi.org/10.3390/polym11091387