Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
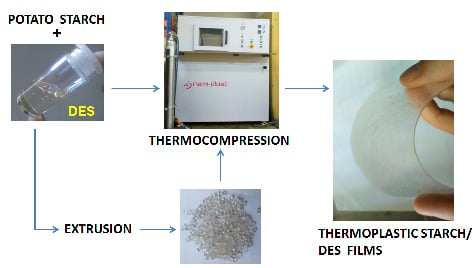
2.2. Preparation of DES
2.3. Preparation of PS/DES Premixtures and TPS/DES Thermocompressed Films
2.4. Preparation of TPS/DES Extrudates
2.5. DSC
2.6. Viscosity Measurement
2.7. Mechanical Tests
2.8. XRD
2.9. DMA Measurement
2.10. FTIR-ATR Analysis
2.11. Electrical Resistivity Measurements
2.12. Moisture Content, Moisture Sorption, and Water Sorption Degrees
2.13. Statistical Analysis
3. Results
3.1. DES Characteristics
3.2. DSC Study of Starch/DES Premixtures
3.3. Mechanical Tests
3.3.1. Mechanical Results for Thermocompressed Films
3.3.2. Mechanical Results for Films Prepared from Extrudates
3.4. XRD of TPS/DES Films Pepared via Two Methods
3.5. DMA Measurements for TPS/DES Films
3.6. FTIR-ATR Analysis
3.7. Electrical Resistivity of TPS/DES Films
3.8. Moisture Content, Moisture Sorption, and Water Sorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, F.; Zhang, B.; Wang, D. Chapter 7-Starch thermal processing: Technologies at laboratory and semi-industrial scales. In Starch Based Materials Food Packaging; Academic Press: Amsterdam, The Netherlands, 2017; pp. 187–227. [Google Scholar]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Schmidt, B.; Spychaj, T. Sorption of Cu2+ and Fe3+ onto starch grafted copolymers obtained via reactive extrusion. Przemysł Chem. 2010, 89, 1628–1630. [Google Scholar]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Da Róz, A.L.; Carvalho, A.J.F.; Gandini, A.; Curvelo, A.A.S. The effect of plasticizers on thermoplastic starch compositions obtained by melt processing. Carbohydr. Polym. 2006, 63, 417–424. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Wilhelm, A.E.; Mali, S.; Grossman, M.V.E. Influence of thickness on properties of plasticized oat starch films. Brazilian Arch. Biol. Technol. 2013, 56, 367–644. [Google Scholar] [CrossRef]
- González, K.; Martin, L.; González, A.; Retegi, A.; Eceiza, A.; Gabilondo, N. D-isosorbide and 1,3-propanediol as plasticizers for starch-based films: Characterization and aging study. J. Appl. Polym. Sci. 2017, 134, 44793. [Google Scholar]
- Ma, X.; Yu, J.; He, K.; Wang, N. The Effects of Different Plasticizers on the Properties of Thermoplastic Starch as Solid Polymer Electrolytes. Macromol. Mater. Eng. 2007, 292, 503–510. [Google Scholar] [CrossRef]
- Mathew, A.P.; Dufresne, A. Plasticized Waxy Maize Starch: Effect of Polyols and Relative Humidity on Material Properties. Biomacromolecules 2002, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Plasticization of Pea Starch Films with Monosaccharides and Polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- Schmitt, H.; Guidez, A.; Prashantha, K.; Soulestin, J.; Lacrampe, M.; Krawczak, P. Studies on the effect of storage time and plasticizers on the structural variations in thermoplastic starch. Carbohydr. Polym. 2015, 115, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Spychaj, T. Ionic liquids: Media for starch dissolution, plasticization and modification. Carbohydr. Polym. 2011, 86, 424–428. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T. Ionic liquids as starch plasticizers or solvents. Polimery 2011, 56, 861–864. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing: A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.D.; Conde, J.P.; Ryder, K.S.; Wise, W.R. Salt modified starch: Sustainable, recyclable plastics. Green Chem. 2012, 14, 1302. [Google Scholar] [CrossRef]
- Adamus, J.; Spychaj, T.; Zdanowicz, M.; Jędrzejewski, R. Thermoplastic starch with deep eutectic solvents and montmorillonite as a base for composite materials. Ind. Crop. Prod. 2018, 123, 278–284. [Google Scholar] [CrossRef]
- Leroy, E.; DeCaen, P.; Jacquet, P.; Coativy, G.; Pontoire, B.; Réguerre, A.-L.; Lourdin, D. Deep eutectic solvents as functional additives for starch based plastics. Green Chem. 2012, 14, 3063. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Exerted influence of deep eutectic solvent concentration in the room temperature ionic conductivity and thermal behavior of corn starch based polymer electrolytes. J. Mol. Liq. 2012, 166, 40–43. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T.; Mąka, H. Imidazole-based deep eutectic solvents for starch dissolution and plasticization. Carbohydr. Polym. 2016, 140, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Johansson, C. Mechanical and barrier properties of starch-based films plasticized with two- or three component deep eutectic solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Johansson, C. Impact of additives on mechanical and barrier properties of starch-based films plasticized with deep eutectic solvents. Starch Stärke 2017, 69, 1700030. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Jędrzejewski, R.; Pilawka, R. Deep eutectic solvents as simultaneous plasticizing and crosslinking agents for starch. Int. J. Biol. Macromol. 2019, 129, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Spronsen van, J.; Witkamp, G.J. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Diarce, G.; Gandarias, I.; Campos-Celador, A.; García-Romero, A.; Griesser, U. Eutectic mixtures of sugar alcohols for thermal energy storage in the 50–90 °C temperature range. Sol. Energy Mater. Sol. Cells 2015, 134, 215–226. [Google Scholar] [CrossRef]
- Maugeri, Z.; De Maria, P.D. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Francisco, M.; Bruinhorst, A.V.D.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R. Optimizing the mechanical and physical properties of thermoplastic starch via tuning the molecular microstructure through co-plasticization by sorbitol and glycerol. Polym. Int. 2017, 66, 809–819. [Google Scholar] [CrossRef]
- Mikus, P.-Y.; Alix, S.; Soulestin, J.; Lacrampe, M.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastic. Ind. Crops Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef]
- Zobel, H.F.; Young, S.N.; Rocca, L.A. Starch gelatinization: An X-ray diffraction study. Cereal Chem. 1988, 65, 443–446. [Google Scholar]
- Hulleman, S.; Helbert, W.; Chanzy, H. Single crystals of V amylose complexed with glycerol. Int. J. Biol. Macromol. 1996, 18, 115–122. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Hanna, M.D. Amylose-lipid complex formation during single-screw extrusion of various corn starches. Cereal Chem. 1994, 71, 582–587. [Google Scholar]
- Putseys, J.A.; Lamberts, L.L.; Delcour, J.A. Amylose inclusion complexes: Formation, identity and physicochemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2010, 119, 2439–2448. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticied thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Willett, J.; Millard, M.; Jasberg, B. Extrusion of waxy maize starch: Melt rheology and molecular weight degradation of amylopectin. Polymer 1997, 38, 5983–5989. [Google Scholar] [CrossRef]
- Liu, W.-C.; Halley, P.J.; Gilbert, R.G. Mechanism of Degradation of Starch, a Highly Branched Polymer, during Extrusion. Macromolecules 2010, 43, 2855–2864. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, X.; Liu, P.; Yu, L.; Li, M.; Zhou, S.; Xie, D.F. Shear degradation of corn starches with different amylose contents. Food Hydrocoll. 2017, 66, 199–205. [Google Scholar] [CrossRef]
- Muller, P.; Kapin, É.; Fekete, E. Effects of preparation methods on the structure and mechanical properties of wet conditioned starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2014, 113, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nessi, V.; Rolland-Sabaté, A.; Lourdin, D.; Jamme, F.; Chevigny, C.; Kansou, V. Multi-scale characterization of thermoplastic starch structure Rusing Second Harmonic Generation imaging and NMR. Carbohydr. Polym. 2018, 194, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Barzic, A.I.; Ioan, S. Multiphase Polymer Systems: Micro-to Nanostructured Evolution in Advanced Technologies; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrrared spectroscopy as tool to characterize starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell Wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Vu, H.P.N.; Lumdubwong, N. Starch behaviors and mechanical properties of starch blend films with different plasticizers. Carbohydr. Polym. 2016, 154, 112–120. [Google Scholar]







| Abbreviation | Description of Sample (Starch/Plasticizer Premixture) Conditioning |
|---|---|
| 1 d | Storage for 24 h in sealed vial at ambient conditions before processing |
| 4 d | Storage for 4 days in sealed vial at ambient conditions before processing |
| f | Processing after (about 30 min) premixture preparation |
| ht | Heating at 80 °C/1h in sealed vial and storage for 1 day before processing |
| ext | Extruded sample |
| DES Acronyms with Components Molar Ratios | Transition Temperature (°C) | Onset Degradation Temperature (°C) | Viscosity at 25 °C/80 °C (mPas) | DES Appearance at Room Temperature |
|---|---|---|---|---|
| X:CC 2:1 | Tg −54.1 | 234.0 | 2420/82 | Colorless, tendency to crystallization |
| X:G 1:2 | Tg −69.1 | 216.0 | 1070/160 | Colorless |
| S:CC 2:1 | Tg −29.1 | 267.6 | 11,500/320 | Slightly yellowish |
| S:CC 1:2 | Tg −53.4 | 235.0 | n.d./130 | Colorless, tendency to crystallization |
| S:G 1:1 | Tg −36.2 | 160.4 | a.d.l./352 | Slightly yellowish |
| S:G 1:2 | Tg −53.4 | 156.0 | 1560/82 | Slightly yellowish |
| S:B 2:1 | Tg −19.1 | 191.1 | n.d. | Yellowish, glassy state |
| M:CC 1:4 | Tm 80.6 | 242.1 | n.d. | Colorless, tendency to crystallization |
| M:G 1:6 | Tg −65.1 | 227.0 | 850/71 | Colorless, tendency to crystallization |
| Sample | PS/DES Premixtures (Pastes Investigated before Thermocompression) | ||
|---|---|---|---|
| To (°C) | Tmin (°C) | ∆H (J/g) | |
| PS/G | 63.2 | 104.0 | 91.1 |
| PS/X | 88.4 a | 93.4 a | 147.5 a |
| PS/X ht | 50.9 | 98.0 | 223.0 |
| PS/X:CC 2:1 | 68.1 | 100.6 | 114.5 |
| PS/X:G 1:2 | 121.4 | 136.3 | 158.1 |
| PS/S | 81.5 a | 94.0 a | 163.0 a |
| PS/S ht | 38.5 | 83.2 | 153.6 |
| PS/S:CC 2:1 | 86.6 | 117.0 | 207.5 |
| PS/S:CC 2:1 ht | 76.5 | 128.9 | 131.2 |
| PS/S:CC 1:2 | 107.4 | 130.4 | 139.4 |
| PS/S:B 2:1 | 81.9 | 118.7 | 320.8 |
| PS/S:B 2:1 ht | 37.3 | 76.1 | 156.1 |
| PS/S:G 1:1 | 114.8 | 126.6 | 210.7 |
| PS/S:G 1:1 ht | 60.8 | 116.2 | 68.4 |
| PS/S:G 2:1 | 112.7 | 134.5 | 140.5 |
| PS/M | 41.9 | 87.3 b | 291.3 |
| PS/M ht | 40.4 | 79.1 b | 186.6 |
| PS/M:CC 1:4 ht | 96.4 | 122.3 | 36.8 |
| PS/M:G 1:6 | 44.2 | 86.3 | 95.2 |
| PS/CC 2d | 91.7 | 129.4 | 285.9 |
| PS/CC 4d | 100.1 | 129.7 | 291.3 |
| Sample | Results for Thermoplastic Starch (TPS)/DES Films Obtained via Thermocompression | ||||
|---|---|---|---|---|---|
| Moisture Content (%) | Tensile Strength (TS) (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Remarks | |
| TPS/G | 10.7 (0.4) | 4.0 (0.10) | 36.5 (4.3) | 135 (11.3) | Transparent, flexible |
| TPS/X | n.m | Increasing crystallization during storage | |||
| TPS/X ht | n.m. | ||||
| TPS/X:CC 2:1 | n.m. | Transparent but brittle | |||
| TPS/X:G 1:2 | 7.6 (0.1) | 6.2 (0.24) | 63.5 (10.2) | 217 (15.1) | Transparent, flexible a |
| TPS/S | n.m. | Brittle/partially plasticized | |||
| TPS/S ht | 5.0 (0.4) | 8.7 (0.42) | 15.1 (2.8) | 451 (83.1) | Unplasticized edges |
| TPS/S:CC 2:1 | 6.1 (0.2) | 8.6 (0.44) | 33.4 (2.0) | 428 (47.6) | Unplasticized edges |
| TPS/S:CC 2:1 ht | 5.9 (0.2) | 7.9 (0.54) | 37.1 (2.7) | 334 (40.9) | Unplasticized edges |
| TPS/S:CC 1:2 | n.m. | Brittle | |||
| TPS/S:B 2:1 | n.m. | Brittle | |||
| TPS/S:B 2:1 ht | 5.2 (0.4) | 8.1 (0.42) | 18.8 (3.7) | 537 (42.4) | Unplasticized edges |
| TPS/S:G 1:1 | n.m. | Partially plasticized, brittle | |||
| TPS/S:G 1:1 ht | 6.2 (1.4) | 7.7 (0.62) | 38.1 (10.6) | 361 (23.5) | Unplasticized edges |
| TPS/S:G 2:1 | 5.7 (0.9) | 6.8 (0.10) | 69.4 (7.6) | 271 (15.1) | Transparent, flexible |
| TPS/M | n.m | Transparent but brittle | |||
| TPS/M ht | n.m | Highly transparent but brittle | |||
| TPS/M:CC 1:4 ht | 9.9 (0.8) | 7.8 (0.16) | 37.3 (6.2) | 354 (24.0) | Transparent |
| TPS/M:G 1:6 | 7.7 (0.7) | 4.9 (0.30) | 40.6 (7.1) | 206 (26.0) | Transparent |
| TPS/CC 2d | n.m. | Partially plasticized, brittle core | |||
| TPS/CC 4d | n.m. | 2.9 (0.35) | 8.5 (2.6) | 205 (27.7) | Weak and matt |
| Sample | Surface Resistivity (Ω·m) | Bulk Resistivity (Ω) |
|---|---|---|
| Thermocompressed | ||
| S:CC | 8.6 × 106 | 4.5 × 104 |
| S:CC ht | 6.0 × 108 | 1.0 × 106 |
| Extruded (100 rpm) | ||
| S:CC f | 9.9 × 106 | 9.1 × 104 |
| S:CC 1d | 7.6 × 106 | 5.0 × 104 |
| S:CC ht | 6.9 × 106 | 4.0 × 104 |
| Sample | Moisture Sorption RH 50% (%) | Moisture Sorption RH 75% (%) | Water Sorption (%) |
|---|---|---|---|
| TPS/G press | 10.3 (0.10) | 30.0 (0.44) | 104.8 (17.7) |
| TPS/S:CC press | 6.2 (0.41) | 24.0 (0.62) | 108.5d (7.3) |
| TPS/G ext | 8.5 (0.21) | 30.5 (0.86) | 78.5 (1.5) |
| TPS/S:CC ext | 5.9 (0.10) | 22.9 (0.36) | 83.2 (1.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdanowicz, M.; Staciwa, P.; Jędrzejewski, R.; Spychaj, T. Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers. Polymers 2019, 11, 1385. https://doi.org/10.3390/polym11091385
Zdanowicz M, Staciwa P, Jędrzejewski R, Spychaj T. Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers. Polymers. 2019; 11(9):1385. https://doi.org/10.3390/polym11091385
Chicago/Turabian StyleZdanowicz, Magdalena, Piotr Staciwa, Roman Jędrzejewski, and Tadeusz Spychaj. 2019. "Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers" Polymers 11, no. 9: 1385. https://doi.org/10.3390/polym11091385