Hydrosilylation of Reactive Quantum Dots and Siloxanes for Stable Quantum Dot Films
Abstract
:1. Introduction
2. Experiment
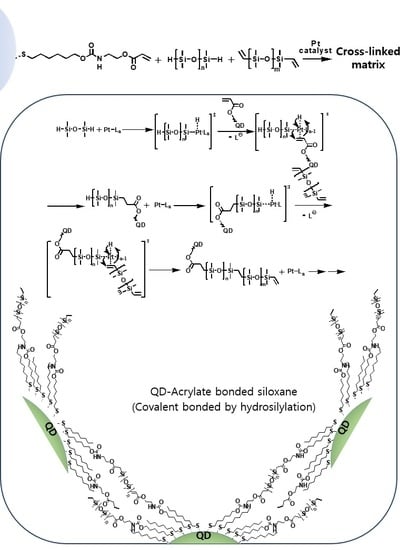
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Sargent, E.H. Solar Cells, Photodetectors, and Optical Sources from Infrared Colloidal Quantum Dots. Adv. Mater. 2008, 20, 3958–3964. [Google Scholar] [CrossRef]
- Trindade, T.; O’Brien, P.; Pickett, N.L. Nanocrystalline Semiconductors: Synthesis, Properties, and Perspectives. Chem. Mater. 2001, 13, 3843–3858. [Google Scholar] [CrossRef]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Bera, D.; Seal, S. One Dimensional Nanostructured Materials. ChemInform 2007, 38, 699–913. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Coe-Sullivan, S. Quantum dot developments. Nat. Photonics 2009, 3, 315–316. [Google Scholar] [CrossRef]
- Lee, J.; Sundar, V.C.; Heine, J.R.; Bawendi, M.G.; Jensen, K.F. Full Color Emission from II–VI Semiconductor Quantum Dot–Polymer Composites. Adv. Mater. 2002, 12, 1102–1105. [Google Scholar] [CrossRef]
- Nozik, A.J. Quantum dot solar cells. Phys. E 2002, 14, 115–120. [Google Scholar] [CrossRef]
- Winiarz, J.G.; Zhang, L.; Park, J.; Prasad, P.N. Inorganic: Organic Hybrid Nanocomposites for Photorefractivity at Communication Wavelengths. J. Phys. Chem. B 2002, 106, 967–970. [Google Scholar] [CrossRef]
- Xiao, P.; Huang, J.; Yan, D.; Luo, D.; Yuan, J.; Liu, B.; Liang, D. Emergence of Nanoplatelet Light-Emitting Diodes. Materials 2018, 11, 1376. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Liu, B.; Qiu, Y. Emergence of Flexible White Organic Light-Emitting Diodes. Polymers 2019, 11, 384. [Google Scholar] [CrossRef]
- Liu, B.; Delikanli, S.; Gao, Y.; DeDe, D.; Gungor, K.; Demir, H.V. Nanocrystal light-emitting diodes based on type II nanoplatelets. Nano Energy 2018, 47, 115–122. [Google Scholar] [CrossRef]
- Mai, R.; Wu, X.; Jiang, Y.; Meng, Y.; Liu, B.; Hu, X.; Roncali, J.; Zhou, G.; Liu, J.-M.; Kempa, K.; et al. An efficient multi-functional material based on polyether-substituted indolocarbazole for perovskite solar cells and solution-processed non-doped OLEDs. J. Mater. Chem. A 2019, 7, 1539–1547. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M.; Frederix, P.L.T.M.; Bol, A.A.; Gerritsen, H.C.; Meijerink, A. Blueing, Bleaching, and Blinking of Single CdSe/ZnS Quantum Dots. ChemPhysChem 2002, 3, 871–879. [Google Scholar] [CrossRef]
- Pechstedt, K.; Whittle, T.; Baumberg, J.; Melvin, T. Photoluminescence of Colloidal CdSe/ZnS Quantum Dots: The Critical Effect of Water Molecules. J. Phys. Chem. C 2010, 114, 12069–12077. [Google Scholar] [CrossRef]
- Carrillo-Carrio’n, C.; Ca´rdenas, S.; Simonet, B.M.; Valcarcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214–5226. [Google Scholar] [CrossRef]
- Bullen, C.; Mulvaney, P. The Effects of Chemisorption on the Luminescence of CdSe Quantum Dots. Langmuir 2006, 22, 3007–3013. [Google Scholar] [CrossRef]
- Zhao, Y.; Riemersma, C.; Pietra, F.; Koole, R.; Donega’, C.; Meijerink, A. High-Temperature Luminescence Quenching of Colloidal Quantum Dots. ACS Nano 2012, 6, 9058–9067. [Google Scholar] [CrossRef]
- Kitai, A. Materials for Solid State Lighting and Displays; John wiley & Sons: Oxford, UK, 2017. [Google Scholar]
- Cao, X.; Li, C.M.; Bao, H.; Bao, Q.; Dong, H. Fabrication of Strongly Fluorescent Quantum Dot−Polymer Composite in Aqueous Solution. Chem. Mater. 2007, 19, 3773–3779. [Google Scholar] [CrossRef]
- Mao, L.-H.; Tang, W.-Q.; Deng, Z.-Y.; Wang, C.-F.; Liu, S.-S.; Chen, S. Facile Access to White Fluorescent Carbon Dots toward Light-Emitting Devices. Ind. Eng. Chem. 2014, 53, 6417–6425. [Google Scholar] [CrossRef]
- Guan, X.; Fan, H.; Jia, T.; Zhang, D.; Lei, Z.; Lai, S. A Versatile Synthetic Approach to Covalent Binding of Polymer Brushes on CdSe/CdS Quantum Dots Surface: Multitype Modification of Nanocrystals. Macromol. Chem. Phys. 2016, 217, 664–671. [Google Scholar] [CrossRef]
- Pereira, A.S.; Rauwel, P.; Reis, M.S.; Silva, N.J.O.; Barros-Timmons, A.; Trindade, T. Polymer encapsulation effects on the magnetism of EuS nanocrystals. J. Mater. Chem. 2008, 18, 4572. [Google Scholar] [CrossRef]
- Neves, M.C.; A Martins, M.; Soares-Santos, P.C.R.; Rauwel, P.; Ferreira, R.A.S.; Monteiro, T.; Carlos, L.D.; Trindade, T. Photoluminescent, transparent and flexible di-ureasil hybrids containing CdSe/ZnS quantum dots. Nanotechnology 2008, 19, 155601. [Google Scholar] [CrossRef]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-Light-Emitting Diodes with Quantum Dot Color Converters for Display Backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef]
- Chen, J.; Hardev, V.; Hartlove, J.; Hofler, J.; Lee, E. A High-Efficiency Wide-Color-Gamut Solid-State Backlight System for LCDs Using Quantum Dot Enhancement Film. SID Int. Symp. Dig. Tech. Pap. 2012, 43, 895–896. [Google Scholar] [CrossRef]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef]
- Bae, W.; Char, K.; Hur, H.; Lee, S. Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem. Mater. 2008, 20, 531–539. [Google Scholar] [CrossRef]
- Zorn, M.; Bae, W.K.; Lee, C.; Char, K. Quantum Dot−Block Copolymer Hybrids with Improved Properties and Their Application to Quantum Dot Light-Emitting Devices. ACS Nano 2009, 3, 1063–1068. [Google Scholar] [CrossRef]
- Anderson, B.D.; Wu, W.-C.; Tracy, J.B. Silica Overcoating of CdSe/CdS Core/Shell Quantum Dot Nanorods with Controlled Morphologies. Chem. Mater. 2016, 28, 4945–4952. [Google Scholar] [CrossRef]
- Farmer, S.C.; Patten, T.E. Photoluminescent Polymer/Quantum Dot Composite Nanoparticles. Chem. Mater. 2001, 13, 3920–3926. [Google Scholar] [CrossRef]
- Dubois, F.; Mahler, B.; Dubertret, B.; Doris, E.; Mioskowski, C. A Versatile Strategy for Quantum Dot Ligand Exchange. J. Am. Chem. Soc. 2007, 129, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Dykstra, T.E.; Salvador, M.R.; Manners, I.; Scholes, G.D.; Winnik, M.A. Surface Passivation of Luminescent Colloidal Quantum Dots with Poly(Dimethylaminoethyl methacrylate) through a Ligand Exchange Process. J. Am. Chem. Soc. 2004, 126, 7784–7785. [Google Scholar] [CrossRef]
- Pong, B.K.; Trout, B.L.; Lee, J. Modified Ligand-Exchange for Efficient Solubilization of CdSe/ZnS Quantum Dots in Water: A Procedure Guided by Computational Studies. Langmuir 2008, 24, 5270–5276. [Google Scholar] [CrossRef]
- Ho, T.-H.; Wang, C.-S. Modification of epoxy resins by hydrosilation for electronic encapsulation application. J. Appl. Polym. Sci. 1994, 54, 13–23. [Google Scholar] [CrossRef]
- Woo, H.; Lim, J.; Lee, Y.; Sung, J.; Shin, H.; Oh, J.M.; Choi, M.; Yoon, H.; Bae, W.K.; Char, K. Robust, processable, and bright quantum dot/organosilicate hybrid films with uniform QD distribution based on thiol-containing organosilicate ligands. J. Mater. Chem. C 2013, 1, 1983–1989. [Google Scholar] [CrossRef]
- Kim, S.; Bawendi, M.G. Oligomeric Ligands for Luminescent and Stable Nanocrystal Quantum Dots. J. Am. Chem. Soc. 2003, 125, 14652–14653. [Google Scholar] [CrossRef]
- Wang, C.-W.; Moffitt, M.G. Surface-Tunable Photoluminescence from Block Copolymer-Stabilized Cadmium Sulfide Quantum Dots. Langmuir 2004, 20, 11784–11796. [Google Scholar] [CrossRef]
- Brien, P.O.; Cummins, S.S.; Darcy, D.; Dearden, A.; Masala, O.; Pickett, N.L.; Ryley, S.; Sutherland, A.J. Quantum dot-labelled polymer beads by suspension polymerisation. Chem. Commun. 2003, 20, 2532–2533. [Google Scholar] [CrossRef]
- Troegel, D.; Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 2011, 255, 1440–1459. [Google Scholar] [CrossRef]
- Gong, Y.-K.; Winnik, F.M. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 2012, 4, 360–368. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnoes, A.M.; Clapp, A.R. Dithiocarbamates as Capping Ligands for Water-Soluble Quantum Dots. ACS Appl. Mater. Interfaces 2010, 2, 3384–3395. [Google Scholar] [CrossRef]
- Marciniec, B. Advances in Silicon Science; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Cicero, R.L.; Linford, M.R.; Chidsey, C.E.D. Photoreactivity of Unsaturated Compounds with Hydrogen-Terminated Silicon(111). Langmuir 2000, 16, 5688–5695. [Google Scholar] [CrossRef]
- Cai, W.; Peck, J.R.; Van Der Weide, D.W.; Hamers, R.J. Direct electrical detection of hybridization at DNA-modified silicon surfaces. Biosens. Bioelectron. 2004, 19, 1013–1019. [Google Scholar] [CrossRef]
- Sommer, L.H.; Lyons, J.E.; Fujimoto, H. Stereochemistry of asymmetric silicon. XV. Stereospecific hydrosilation and exchange reactions of R3SiH(D) catalyzed by Group VIII metal centers. J. Am. Chem. Soc. 1969, 91, 7051–7061. [Google Scholar] [CrossRef]
- Sakaki, S.; Mizoe, N.; Sugimoto, M. Theoretical Study of Platinum(0)-Catalyzed Hydrosilylation of Ethylene. Chalk−Harrod Mechanism or Modified Chalk−Harrod Mechanism. Organometallics 1998, 17, 2510–2523. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Catala, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004, 20, 1–7. [Google Scholar] [CrossRef]
- Kjeldsen, P. Evaluation of gas diffusion through plastic materials used in experimental and sampling equipment. Water Res. 1993, 27, 121–131. [Google Scholar] [CrossRef]
- Velderrain, M. Designing low permeability, optical-grade silicone systems: Guidelines for choosing a silicone based on transmission rates for barrier applications. SPIE OPTO 2012, 8280, 82800. [Google Scholar]








| Supplied Chemicals | 6-Mercaptohexanol | 2-Isocyanatoethyl acrylate | 1-Octanethiol |
|---|---|---|---|
| Supplied amounts (mmol) | 29.2 | 29.2 | 46.1 |
| mol ratio | 1.00 | 1.00 | 1.58 |
| No. of protons (position) | 2 (Adjacent protons to the hydroxy group) | 3 (protons of the acrylate group) | 3 (protons of the terminal carbon) |
| Integrated value (by 1H-NMR) | 2.00 (The peak at 3.6 ppm in Figure 2a) | 3.00 (The peaks from 6 to 6.5 ppm in Figure 2b) | 4.75 (The peak at 0.9 ppm in Figure 2a,b) |
| Calculated value (by supplied chemicals) | 1.00 × 2 = 2.00 | 1.00 × 3 = 3 | 1.58 × 3 = 4.74 |
| QY * (%) | Full Width at Half Maximums (FWHM) ** (nm) | Emission max (nm) | |
|---|---|---|---|
| Oleic-acid-coordinated QDs (QD-OA) | 91 | 20 | 525 |
| QD-OH | 94 | 21 | 527 |
| QD-Acrylate | 93 | 20 | 527 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Nam, E.; Lee, W.; Chae, H. Hydrosilylation of Reactive Quantum Dots and Siloxanes for Stable Quantum Dot Films. Polymers 2019, 11, 905. https://doi.org/10.3390/polym11050905
Lee C, Nam E, Lee W, Chae H. Hydrosilylation of Reactive Quantum Dots and Siloxanes for Stable Quantum Dot Films. Polymers. 2019; 11(5):905. https://doi.org/10.3390/polym11050905
Chicago/Turabian StyleLee, Changmin, Eunhee Nam, Woosuk Lee, and Heeyeop Chae. 2019. "Hydrosilylation of Reactive Quantum Dots and Siloxanes for Stable Quantum Dot Films" Polymers 11, no. 5: 905. https://doi.org/10.3390/polym11050905