1. Introduction
Nitric acid is mainly used for producing nitrogen fertilizers: ammonium nitrate (AN) and calcium ammonium nitrate (CAN) which constitute 75–80% of its entire production. The remaining amount of nitric acid is used in other industrial applications for example as a nitration agent for the production of explosives and other semi-organic products (aliphatic nitro compounds and aromatic nitro compounds) for the production of adipic acid, for metallurgy (etching steel) [
1].
The industrial production of nitric acid is based on Ostwald process [
1] which involves three basic stages: the catalytic oxidation of ammonia to nitrogen oxide (NO) with the use of oxygen from air, oxidation of nitrogen oxide (NO) to nitrogen dioxide (NO
2) and absorption of nitrogen oxides in water with the formation of HNO
3.
Ammonia consumption depends on the selectivity of the applied ammonia oxidation catalyst and on the process conditions. Among numerous catalysts [
2,
3,
4,
5,
6,
7,
8], packages of gauzes made of noble metal alloys such as platinum and rhodium are most commonly applied in industrial practice [
7,
8,
9,
10]. Properly selected catalyst package allows to obtain ammonia conversion to main product (NO) in the range of 90–98% depending mainly on oxidation pressure [
1,
7,
8]. Oxidation pressure has an inversely proportional effect on ammonia oxidation efficiency. In order to alleviate this effect, the temperature of reaction should be higher. However, this leads to the increased platinum losses and as a consequence, shortens the lifetime of the catalytic gauzes. For example, platinum losses are six times higher after increasing the temperature of reaction from 820 to 920 °C [
1,
2]. Therefore, both these aspects should be taken into account to determine the temperature of reaction.
The application of medium pressure in the oxidation unit (0.35–0.55 MPa) and high pressure in the absorption unit (0.8–1.5 MPa) is optimal for specific ammonia consumption and efficient energy use. Therefore, modern nitric acid plants are dual-pressure ones. The average pressure in the oxidation unit is a kind of trade-off between the capacity that is possible to achieve per 1 m
2 of catalytic gauzes, oxidation efficiency, number of gauzes in package, lifetime of gauzes and noble metals losses during exploitation [
1,
2].
In the context of global warming and climate changes, a very important issue related to ammonia oxidation process is the amount of the by-product formed that is nitrous oxide (N
2O). In Kyoto Protocol, N
2O was qualified as a greenhouse gas with a very high global warming potential, about 300 times higher than CO
2 [
11]. At room temperature, N
2O is a colourless, non-flammable gas with a delicate pleasant smell and sweet taste [
12]. Since it was isolated at the end of 17th century and because of its pain-relieving and anaesthetic properties, it has been widely applied in dentistry and surgery. Currently, due to some concerns, there is an ongoing discussion on its safe use which has the effect of decreasing the N
2O application in medicine [
12,
13]. At the same time, the increasing trend of its use for recreational purposes is observed. Inhaling the ‘laughing gas’ causes euphoria and hallucinations [
13].
Microbial nitrification and de-nitrification in land and aqueous eco-systems are the natural sources of N
2O in environment. The anthropogenic sources are cultivated soils fertilized intensely with nitrogen fertilizers and industrial processes such as burning fossil fuels and biomass as well as the production of adipic acid and nitric acid with the last one being regarded as the biggest source of N
2O in the chemical industry [
14,
15]. Nitrous oxide formed in nitric acid plant does not undergo any conversions and it is released to atmosphere. Currently, the emission of this gas is monitored and industry is obliged to reduce it. Pursuant to BAT requirements, concentration of this gas in outlet gases cannot exceed 20–300 ppm depending on the type of nitric acid plants [
16,
17]. However, due to the battle against climate change and global warming, further restrictions in emission limits can be expected.
There are several methods of limiting N
2O emissions from nitric acid plants [
14]. Generally, they can be classified as primary and secondary methods. Primary methods involve preventing the formation of N
2O during ammonia oxidation. They include modification of catalytic gauzes (so-called low-emissions systems) and parameters optimization of ammonia oxidation process. Secondary methods involve the removal of N
2O. At the temperature over 800 °C, thermal decomposition of N
2O occurs but the efficient decomposition requires ensuring adequately long residence time at high temperatures [
14,
15].
The achievement of low level of N2O emissions requires the application of the catalytic methods such as high temperature N2O decomposition from nitrous gases, low- or middle temperature N2O decomposition or reduction from tail gases. High temperature method is more common. In some cases, the combination of primary method (application of modified catalytic gauzes packages and/or optimization of ammonia oxidation parameters) and high temperature N2O decomposition ensures meeting the emission standards.
Optimization of production process requires extensive knowledge and understanding the effect of particular parameters on the process. Until recently, the most commonly applied approach of researchers to study simple and complex processes was ‘one-factor-at-a-time’ (
OFAT), which is time consuming and ineffective method for processes with multiple complicated dependencies between parameters. Over the last years, mathematical and statistical methods for design of experiments and parameters optimization have been applied more frequently [
18]. Because of its usability, this method is applied for the design, improvement and optimization of production processes and products [
19,
20,
21]. It is a widely applied method in research of various processes [
22,
23,
24,
25,
26,
27] and approx. 50% of all applications is in medicine, engineering, biochemistry, physics and computer science [
28]. In this method, reaction kinetics equations and process mechanism are not taken into account and they are regarded as a ‘black-box’ [
29] (
Figure 1).
The choice of experiment plan depends mainly on the issue which is the subject matter of investigations as well as on objectives which are set. The most commonly applied experiments plans include: full or fractional factorial, Plackett-Burman, central composite, Box-Behnken and Taguchi designs.
As a result of modelling of the data obtained, empirical equations with statistically significant importance are received which describe the effect of process variables (independent variables) on the process result (response variable).
Desirability function (
DF) can be applied in search for optimal operational parameters. The method proposed by Derringer and Suich [
18] involves the construction response surface model and then finding the values of independent variables which ensure the most desirable value. The objective of the presented studies was the analysis of the impact of reactor’s operational parameters on ammonia oxidation reaction. To the best of our knowledge, the approach presented here to describe ammonia oxidation process is published for the first time.
2. Results and Discussion
2.1. Design of Experiments
Ammonia oxidation reaction depends on a few process variables. In this study, the effect of the reactor’s load (
X1), the temperature of nitrous gas specifying the temperature of reaction (
X2) and the number of catalytic gauzes (
X3) on ammonia oxidation reaction was investigated. The oxidation efficiency of NH
3 to NO (
R1) and N
2O concentration in nitrous gases (
R2) were selected as measures for ammonia oxidation reaction. The matrix of 15 experiments including particular levels of coded variables and achieved values of response variables
R1 and
R2 are presented in
Table 1. In the regarded experimental area of independent variables, ammonia oxidation efficiency ranged from 91.4% to 96.4%, whereas N
2O concentration in nitrous gases ranged from 1011 to 1762 ppm.
2.2. Model Fitting
The first task was to find out which equation would allow to obtain the best correlation between independent variables and responses. Analysis of Variance (ANOVA) was carried out for most frequently applied equations: linear, two-factor interaction (2FI), quadratic and cubic.
Table 2 includes the summary statistics of both responses for different mathematical equations.
Based on the achieved results, it was found that the experimental data is described best with quadratic and cubic equations. For both responses, high values of R2 and adjusted R2 were achieved. The number of conducted experiments caused that the cubic model was aliased. It means that the experimental matrix contains an insufficient number of experimental points for independent estimation of all effects for these models. Therefore, quadratic equation was selected for further analysis.
The statistical significance of these equations and their particular terms was specified based on Analysis of Variance (ANOVA). Results of this analysis are presented in
Table 3 and
Table 4, for response variables
R1 and
R2 respectively. Large
F-value indicates that most changes of independent variable can be explained with the developed regression equation. The correlated probability
p-value is used to estimate whether
F-value is large enough to show statistical significance.
The probability p-value for the achieved model of variable R1 is 0.0001. It means that the model is statistically significant but some terms of equation are statistically not significant. Coefficients: R2, adjusted R2 and predicted R2 are very high: 0.9908, 0.9744 and 0.8577, respectively. There is also high compliance between coefficients: predicted R2 and adjusted R2 (difference <0.2). The achievement of statistically significant value lack of fit (0.0256) is the incompliance of this model as this parameter should be statistically not significant.
In case of response variable R2, the probability p-value (0.0012) indicates that the assumed quadratic equation is statistically significant but some of its terms are statistically not significant. High coefficients R2, adjusted R2 and predicted R2 are also achieved for the second response variable and they are: 0.9784, 0.9396 and 0.6574, respectively. However, the difference between predicted R2 and adjusted R2 is larger than the recommended one (>0.2). This may demonstrate a large block effect or problems with model or data. This model is also characteristic of statistically significant parameter lack of fit (p = 0.0119).
At a further stage of analysis, statistically not significant terms of initial equation were eliminated from the analysis. The reduction was made using step-by-step method (from the most insignificant term). For both these response variables, only statistically significant terms were left and higher
R2, adjusted
R2 and predicted
R2 coefficients were achieved. Results of Analysis of Variance (ANOVA) are presented in
Table 5 and
Table 6.
The obtained mathematical model for response R1 is highly significant (p-value < 0.0001). The dependence on linear terms X1, X3, interaction X1X3 and quadratic term X32 are significant. High determination coefficients are obtained l (R2 = 0.9835, adjusted R2 = 0.9768, predicted R2 = 0.9550).
The final model is presented in Equation (1).
The obtained mathematical model for response
R2 is highly significant (
p-value < 0.0001). The dependence on linear terms
X1,
X2,
X3 and quadratic term
X32 are significant. High determination coefficients (
R2 = 0.9513, adjusted
R2 = 0.9318, predicted
R2 = 0.8743) were achieved for the model. The final model is presented in Equation (2).
2.3. Model Diagnostics
Before the process optimization, the model diagnostics for both equations was performed because of occurrence of statistically significant Lack of fit parameter. Results of model diagnostics: a normal probability of the residuals, residuals analysis and actual data versus predicted values plots were analysed.
Figure 2 presents model diagnostics for response variable
R1, whereas
Figure 3 presents model diagnostics for response variable
R2. Normal plot of studentised residuals should be approximately a straight line, whereas studentised residuals versus predicted response values and versus run should be a random scatter. Points in plots of real response values with reference to predicted response values line up accurately along the axis at the angle of 45°.
These diagnostics show that despite the fact that Lack of fit parameter is statistically significant, experimental and predicted points for both equations correlate well with each other.
2.4. The Effect of Independent Variables
In case of response variable
R1 (ammonia oxidation efficiency), the mathematical model shows a strong linear effect of the reactor’s load (
X1) and the number of catalytic gauzes (
X3) and interaction between these two variables (
X1X3) and the quadratic term number of catalytic gauzes (
X32) on the achieved response variable. The temperature of reaction in the studied range does not affect the ammonia oxidation efficiency. The effect of
X1 and
X3 on response
R1 were shown as contour plot (
Figure 4). According to the presented plot of variable of
R1, a small number of catalytic gauzes causes lower ammonia oxidation efficiency for the entire range of studies reactor’s load. The increase in the number of catalytic gauzes to
X3 = 0 causes increase in oxidation efficiency within the entire range of studied reactor’s load.
Studies related to dependency of N
2O concentration in nitrous gases on operating parameters are relatively new research issue. Therefore, there is a lack of scientific reports dedicated to systematic studies in this field. In case of the N
2O concentration in nitrous gases, the achieved mathematical model demonstrates a significant effect of the selected process variables (
X1,
X2,
X3) and the quadratic term number of catalytic gauzes (
X32) on the achieved response variable. This effect is illustrated in
Figure 5,
Figure 6 and
Figure 7. The analysis of Equation (2) and
Figure 5,
Figure 6 and
Figure 7 indicates that the temperature of reaction has the biggest quantitative effect on N
2O concentration in nitrous gases. From the comparison of plots (
Figure 5a–c) it can be concluded that despite the presence of statistically significant terms of equations derived from variable
X3, plots of contour line corresponding to levels 0 and 1 are similar. Only for
X3 = −1, higher values of
R2 are achieved. Profiles of response variable
R2 presented in
Figure 6a–c and
Figure 7a–c confirm the effect of the number of catalytic gauzes. Both these figures show that the number of catalytic gauzes has little effect on the amount of N
2O being formed. For the level of
X3 = 0–0.4 (10–12 gauzes), the local optimum is observed. For this number of gauzes, increasing the reactor’s load (
X1 = 1) at the fixed reaction temperature (
Figure 6a–c) and decreasing the reaction temperature at the fixed reactor’s load (
Figure 7a–c) does not cause a significant decrease in N
2O concentration in nitrous gases.
2.5. Multi-Response Desirability Optimization
The major optimization task is to find the number of catalytic gauzes and the permissible reactor’s load ensuring the maximization of ammonia oxidation efficiency (
R1) and the minimization of N
2O concentration in nitrous gases. Results of experiments discussed in
Section 2.4. indicate that statistically, the temperature has no significant effect on ammonia oxidation efficiency but on the other hand, the amount of N
2O formed is reversely proportional to the temperature of reaction. For desirability function, it was assumed that independent variables are in the variability range. Assumptions for the optimization are presented in
Table 7.
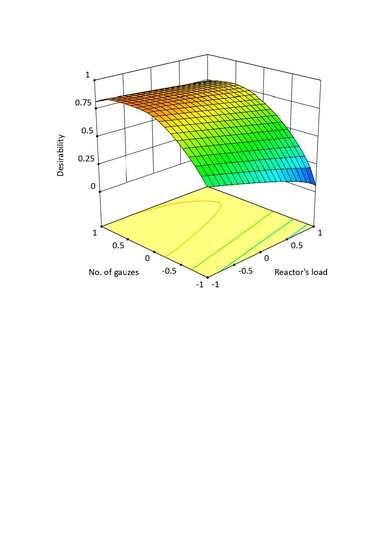
For such optimization assumptions, the area of detailed set of parameters was achieved. It confirms that optimization assumptions are met. Desirability functions for three temperature levels are presented as contour plot in
Figure 8.
High values of desirability function (
DF > 0.9) at 910 °C are described with dependency according to which for the load of 456 kg NH
3/(m
2h), the sufficient number of catalytic gauzes is 8. However, for the maximum load studied, 10 catalytic gauzes should be applied. At the temperature of 910 °C and when all optimization criteria are met, the expected value of N
2O concentration ranges from 1000 ppm to 1100 ppm (
Figure 8a). Lowering the reaction temperature to 890 °C means that desirability function
DF > 0.8 is within the region where the minimum catalyst gauzes is 9 for the loading not higher than 480 kg NH
3/(m
2h) and 12 gauzes for load of 645 kg NH
3/(m
2h) (
Figure 8b). At this temperature, the expected concentration of N
2O in nitrous gases ranges from 1180 to 1200 ppm. At the lowest temperature (within the studied range) of 870 °C, the highest value of desirability function is 0.69. For 12 gauzes and the load of 456 kg/(m
2h), the expected concentration of N
2O in nitrous gases is 1400 ppm.
Taking into account the amount of the primary emissions of N
2O (the environmental aspect), it is favourable to conduct the reaction at the temperature of 910 °C. However, this leads to the increased platinum losses. Platinum losses at 910 °C are higher by approx. 25% as compared to losses at 890 °C and by 45% as compared to losses at 870 °C [
1,
2]. Lowering the reaction temperature to 890 °C with maintaining the optimal range of other parameters causes the increase of N
2O concentration in nitrous gases by 100–200 ppm.
The assumption of other values of ‘weight’ and ‘importance’ for particular variables leads to obtain other profiles of desirability function. Under industrial conditions, the assumed value of ‘weight’ and ‘importance’ should take into account the process economics with regard to platinum losses.
2.6. Validation
Validation of the developed optimization model should be carried out under conditions specified as optimal. Optimization results indicate a wide set of parameters for which desirability function achieved high values. Therefore, in order to carry out additional measurements, the point with the independent variables value of: 1, 1, 1 was selected. This point is in the range of high desirability function value. In
Table 8 levels of independent variables, results of validation experiment and predicted mean values of response variables with standard deviation are presented.
For the assumed independent variables, the values of predicted mean with 95% two-sided confidence intervals met by 99% of population were estimated based on the achieved mathematical model. High conformity of results expected according to mathematical models with the obtained measurements results was achieved.
4. Conclusions
The conducted studies allowed us to develop statistically significant mathematical models describing the course of variables of ammonia oxidation efficiency and N2O concentration in nitrous gases depending on three selected independent variables.
The design of the experiment allowed the reduction of the costs of studies and to achieve a number of results accurate for modelling. It was found that, within the studied range of variability, the temperature of reaction has no significant effect statistically on the achieved ammonia oxidation efficiency, whereas it has the effect on the amount of N2O formed in the side reaction (primary emission of N2O).
The developed models were used to optimize the process. As a result of this optimization, the set of the independent variables was developed for which optimization assumptions are met, which are expressed as a high value of desirability functions. It is possible to specify the optimum number of gauzes with the determined reactor’s load for the studied package of catalytic gauzes.
In validation experiments, the developed model of desirability function achieved the high conformity of experimental values with the expected ones.
The presented methodology can be used to minimize the primary N2O emission at high ammonia oxidation efficiency. It can be applied for optimization of operating parameters of ammonia oxidation reactor with two types of catalysts: catalytic gauzes and catalyst for high temperature of N2O decomposition. As a result, it is possible to obtain the set of independent variables ensuring low N2O emission and to meet the binding environmental regulations.