Heterogeneous Nanomagnetic Catalyst from Cupriferous Mineral Processing Gangue for the Production of Biodiesel
Abstract
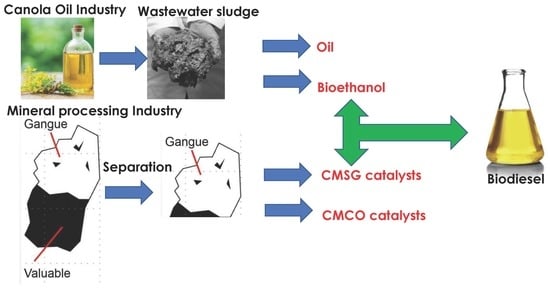
:1. Introduction
2. Results and Discussion
2.1. Gangue Characterisation
2.2. Brunauer-Emmett-Teller (BET) Surface Area Analysis and Barrett-Joyner-Halenda (BJH) Pore Size and Volume Analysis
2.3. Microscopic Observations
2.4. Magnetic Susceptibility and Mass Magnetisation Calculations
2.5. Evaluation of Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Sol-gel Method
3.2.2. Co-precipitation Method
3.2.3. Catalyst Mixed with Zero-Valent Iron Nanoparticles (ZVINPs)
3.3. Biodiesel Production and Separation
3.4. Recovery of Catalyst from Biodiesel
3.5. Catalyst Characterisation and Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabri, L.J.; Rudashevsky, N.S.; Rudashevsky, V.N. Current approaches for the process mineralogy of platinum-group element ores and tailings. In Proceedings of the Ninth International Congress for Applied Mineralogy (ICAM 2008), Kerala State, India, 18–21 February 2008; Volume 8, pp. 9–17. [Google Scholar] [CrossRef] [Green Version]
- Arens, V.Z.; Chernyak, S.A. Hydrometallurgy in the mining industry. Metallurgist 2008, 52, 3–10. [Google Scholar] [CrossRef]
- Cairncross, B. History of the Okiep Copper District, Namaqualand, Northern Cape Province, South Africa. Mineral. Rec. 2004, 35, 289–317. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel Fuels from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Alcohol Transesterifications and Other Methods: A Survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Chen, S.Y.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y. Ti-incorporated SBA-15 mesoporous silica as an efficient and robust Lewis solid acid catalyst for the production of high-quality biodiesel fuels. Appl. Catal. B Environ. 2014, 148, 344–356. [Google Scholar] [CrossRef]
- Hu, S.; Guan, H.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2688. [Google Scholar] [CrossRef]
- Montero, J.M.; Brown, R.; Gai, P.L.; Lee, A.F.; Wilsonc, K. In situ studies of structure-reactivity relations in biodiesel synthesis over nanocrystalline MgO. Chem. Eng. J. 2010, 161, 332–336. [Google Scholar] [CrossRef]
- Amani, H.; Ahmad, Z.; Hameed, B.H. Synthesis of fatty acid methyl esters via the methanolysis of palm oil over Ca3.5xZr0.5yAlxO3 mixed oxide catalyst. Renew. Energy 2014, 66, 680–685. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Elfimov, I.S.; Yunoki, S.; Sawatzky, G.A. Possible path to a new class of ferromagnetic and half-metallic ferromagnetic materials. Phys. Rev. Lett. 2002, 89, 216403. [Google Scholar] [CrossRef] [Green Version]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy. Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Konaka, A.; Tago, T.; Yoshikawa, T.; Shitara, H.; Nakasaka, Y.; Masuda, T. Conversion of biodiesel-derived crude glycerol into useful chemicals over a zirconia–iron oxide catalyst. Ind. Eng. Chem. Res. 2013, 52, 15509–15515. [Google Scholar] [CrossRef]
- Bobade, V.V.; Kulkarni, K.S.; Kulkarni, A.D. Application of Heterogeneous Catalyst for the Production of Biodiesel. Int. J. Adv. Eng. Technol. 2011, 2, 184–185. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yu, K.M.K.; Tam, K.Y.; Tsang, S.C. Colloidal stable silica encapsulated Nanomagnetic composite as a novel bio-catalyst carrier. Chem. Commun. 2003, 24, 2998. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.B.; Wang, Y.; Lu, D.L.; Hu, S.Y.; Han, H.Y. Preparation of KF/CaO nanocatalyst and its application in biodiesel production from Chinese tallow seed oil. Fuels 2010, 89, 2267. [Google Scholar] [CrossRef]
- Ying, M.; Chen, G. Study on the production of biodiesel by magnetic cell biocatalyst based on lipase-producing Bacillus subtilis. Appl. Biochem. Biotechnol. 2007, 137, 793–803. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Hydary, I.A.; Al-Hattab, T.A. Nano-Magnetic Catalyst CaO-Fe3O4 for Biodiesel Production from Date Palm Seed Oil. Bull. Chem. React. Eng. Catal. 2017, 12, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for Biodiesel production. Energy Fuels 2008, 207, 17–22. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Teo, S.H.; Rashid, U.; Islam, A.; Hussien, M.Z.; Lee, K.T. Transesterification of Jatropha curcas crude oil to biodiesel on calcium lanthanum mixed oxide catalyst: Effect of stoichiometric composition. Energy Convers. Manag. 2014, 88, 1290–1296. [Google Scholar] [CrossRef]
- Kouzu, M.; Kajita, A.; Fujimori, A. Catalytic activity of calcined scallop shell for rapeseed oil transesterification to produce biodiesel. Fuels 2016, 182, 220–226. [Google Scholar] [CrossRef]
- Marinkovic, D.M.; Avramovic’, J.M.; Stankovic, M.V.; Stamenkovic, O.S.; Jovanovića, D.M.; Veljković, V.B. Synthesis and characterization of spherically-shaped CaO/γ-Al2O3 catalyst and its application in biodiesel production. Energy Convers. Manag. 2017, 144, 399–413. [Google Scholar] [CrossRef]
- Ngoie, I.W.; Welz, P.J.; Oyekola, O.O.; Ikhu-Omoregbe, D. Valorisation of edible oil wastewater sludge: Bioethanol and biodiesel production. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Ngoie, W.I.; Welz, P.J.; Oyekola, O.O.; Ikhu-Omoregbe, D.I. Qualitative Assessment of Biodiesel Produced from Primary Edible Oil Wastewater Sludge. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Di Serio, M.; Cozzolino, M.; Giordano, M.; Tesser, R.; Patrono, P.; Santacesaria, E. From homogeneous to heterogeneous catalysts in biodiesel production. Ind. Eng. Chem. Res. 2007, 46, 6379–6384. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Duan, Y.; Zhao, C. Reactivity enhancement of calcium based sorbents by doped with metal oxides through the sol-gel process. Appl. Energy 2016, 162, 390–400. [Google Scholar] [CrossRef]
- Coulson, J.M.; Richardson, J.F. Coulson’s and Richardson’s Chemical Engineering Handbook; Chhabra, R.P., Gurappa, B., Eds.; Oxford: Butterworth-Heinemann, UK, 2002. [Google Scholar]
- Rashtizadeh, E.; Farzaneh, F.; Talebpour, Z. Synthesis and characterization of Sr3Al2O6 nanocomposite as catalyst for biodiesel production. Bioresour. Technol. 2014, 154, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO-Fe2O3 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Xie, W.; Fan, M. Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem. Eng. J. 2014, 239, 60–67. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Biodiesel production from crude Jatropha Curcas oil using calcium based mixed oxide catalysts. Fuels 2014, 136, 244–252. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Taufiq-Yap, Y.H. Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuels 2014, 142, 38–45. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schiith, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Cheminternationale 2007, 46, 1222. [Google Scholar] [CrossRef] [PubMed]
- Vishal, T. Biodiesel—An Alternative Method for Energy Crisis: A Review. J. Biol. Chem. Chron. 2016, 2, 14–26. [Google Scholar] [CrossRef]
- Devarapaga, M.; Chavan, S.; Singh, V.; Singh, B.; Sharma, Y.C. An economically viable synthesis of biodiesel from a crude Millettia pinnata oil of Jharkhand, India as feedstock and crab shell derived catalyst. Bioresour. Technol. 2016, 214, 210–217. [Google Scholar] [CrossRef]
- Mayvan, A.A.; Ghobadian, B.; Najafi, G. Electrostatic coagulation for separation of crude glycerin from biodiesel. Adv. Environ. Biol. 2014, 8, 321–324. [Google Scholar]





| Sample | Surface Area (m2/g) | Average Pore Size (Å) | References |
|---|---|---|---|
| CMCO2 * | 52.6 ± 0.37 | 135.6 ± 0.13 | This study |
| CMSG3 * | 58.7 ± 0.55 | 169.3 ± 0.21 | This study |
| CMSG3/ZVINPs2 * | 53.5 ± 1.52 | 148.5 ± 0.09 | This study |
| CaO/Fe3O4 | 59.1 | 8.5 | [26] |
| KF/Ca-Mg-Al hydrotalcite | 108.4 | 3.7 | [14] |
| Catalysts | Average Mass Magnetisation (emu/g) |
|---|---|
| CMCO1 | 153 ± 0.71 |
| CMCO2 | 168 ± 1.45 |
| CMCO3 | 159 ± 0.97 |
| CMSG1 | 76 ± 1.94 |
| CMSG2 | 91 ± 1.06 |
| CMSG3 | 84 ± 0.56 |
| CMSG3/ZVINPs1 | 134 ± 2.52 |
| CMSG3/ZVINPs2 | 173 ± 0.36 |
| CMSG3/ZVINPs3 | 162 ± 1.27 |
| Properties | Unit | Measurement Standards | Commercial Diesel (50ppm Sulphur) | Biodiesel [23] |
|---|---|---|---|---|
| Viscosity at 40 °C | m2/s | ASTM D445 | 3.0 * 10−6 ± 0.87 | 3.7 * 10−6 ± 0.71 |
| Density at 15 °C | kg/m3 | ASTM D941 | 830.0 ± 0.78 | 832.62 ± 0.69 |
| HHV | MJ/kg | ASTM D2015 | 48.12 ± 1.59 | 45.75 ± 1.21 |
| Flash point | °C | ASTM D93 | 50.4 ± 0.73 | 61.3 ± 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoie, W.I.; Welz, P.J.; Ikhu-Omoregbe, D.; Oyekola, O.O. Heterogeneous Nanomagnetic Catalyst from Cupriferous Mineral Processing Gangue for the Production of Biodiesel. Catalysts 2019, 9, 1047. https://doi.org/10.3390/catal9121047
Ngoie WI, Welz PJ, Ikhu-Omoregbe D, Oyekola OO. Heterogeneous Nanomagnetic Catalyst from Cupriferous Mineral Processing Gangue for the Production of Biodiesel. Catalysts. 2019; 9(12):1047. https://doi.org/10.3390/catal9121047
Chicago/Turabian StyleNgoie, Wighens I., Pamela J. Welz, Daniel Ikhu-Omoregbe, and Oluwaseun O. Oyekola. 2019. "Heterogeneous Nanomagnetic Catalyst from Cupriferous Mineral Processing Gangue for the Production of Biodiesel" Catalysts 9, no. 12: 1047. https://doi.org/10.3390/catal9121047