Removal of Banana Tree Fungi Using Green Tuff Rock Powder Waste Containing Zeolite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calcined Green Tuff Powder
2.2. Radical Measurement by Adding Green Tuff and Calcined Powder
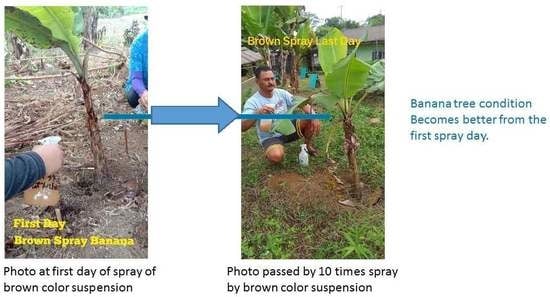
2.3. Result of Infected Banana Trees after about One Month
3. Materials and Experiment
3.1. Green Tuff Powder Characteristics
3.2. Radical Measurement by Electron Spin Resonance (ESR)
3.3. Fusarium Wilt and Experimental Method in a Banana Plants in Luzon Island, Philippines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green Tuff. 2013. Available online: http://www.oki-geopark.jp/en/episode/geohistory/stage2/green-tuff/ (accessed on 1 December 2019).
- Towada Green-Tuff AgroScience Co., Ltd. 2018. Available online: https://towadagreentuff.com/company.html (accessed on 1 December 2019).
- Mitsumori, H.; Aiba, N.; Saito, M.; Nagasawa, Y. A Study on the Use of Towada Stones in Food Processing. Bull. Seirei Women’s J. Coll. 2011, 39, 14–25. [Google Scholar] [CrossRef]
- Sugai, Y.; Sasaki, K.; Matsubaya, O.; Naka, H.; Tanaka, F. The abilities of Hinai-Green Tuff to adjust pH and activate of microorganisms. Shigen-To-Sozai 2005, 121, 513–520. [Google Scholar] [CrossRef]
- Nakamura, T.; Okawa, H.; Kawamura, Y.; Takahata, S.; Naka, H.; Sugawara, K. Research on the New Method to Precipitate Hinai Green Tuff Suspension using Ultrasound Irradiation. Resour. Process. 2009, 56, 13–203. [Google Scholar] [CrossRef]
- Sugai, Y.; Sasaki, K.; Takahata, S.; Naka, H. Characteristics of functional wall material containing a green tuff. Soc. Heat. Air-Cond. Sanit. Eng. Jpn. 2007, 32, 1–10. [Google Scholar] [CrossRef]
- Fujita, T.; Zhang, L.; Dodbiba, G.; Ahn, J.W.; Wei, Y.; Kukrokawa, H.; Matsui, H.; Yamamoto, S.; Kawaguchi, H. Production of the hydroxyl radical and removal of calcined green tuff powder and tile. Sustainability 2019, 11, 3390. [Google Scholar] [CrossRef] [Green Version]
- Wajima, T. Synthesis of zeolitic material from green tuff stone cake and its adsorption properties of silver (I) from aqueous solution. Microporous Mesoporous Mater. 2016, 223, 154–162. [Google Scholar] [CrossRef]
- Humelnicua, I.; Baiceanu, A.; Ignat, M.E.; Dulman, V. The removal of Basic Blue 41 textile dye fromaqueous solution by adsorption onto naturalzeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Bozcheloei, K.V.; Tangestani, M.H. Prospecting for Clinoptilolite-Type Zeolite in a Volcano-Sedimentary Terrain Using ASTER Data: A Case Study from Alborz Mountains, Northern Iran. Nat. Resour. Res. 2019, 28, 1317–1327. [Google Scholar] [CrossRef]
- Davari, N.; Farhadian, M.; Nazar, A.R.S.; Homayoonfal, M. Degradation of diphenhydramine by the photocatalysis of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J. Environ. Chem. Eng. 2017, 5, 5707–5720. [Google Scholar] [CrossRef]
- Jaffri, S.B.; Ahmad, K.S. Neoteric environmental detoxification of organic pollutants and pathogenic microbes via green synthesized ZnO nanoparticles. Environ. Technol. 2019, 40, 3745–3761. [Google Scholar] [CrossRef]
- Labuz, P.; Grybos, J.; Pietrzyk, P.; Sobanska, K.; Macyk, W.; Sojka, Z. Photogeneration of reactive oxygen species over ultrafine TiO2 particles functionalized with rutin-ligand induced sensitization and crystallization effects. Res. Chem. Int. 2019, 45, 5781–5800. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Choi, H.; Kushida, Y.; Bhayana, B.; Wang, Y.; Hamblina, M.R. Broad-Spectrum Antimicrobial Effects of Photocatalysis Using Titanium Dioxide Nanoparticles Are Strongly Potentiated by Additio of Potassium Iodide. Antimicrob. Agent. Chemother. 2016, 60, 5445–5453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.Y.; Wang, L.; Wang, H.; Liu, Z.Y.; Xing, M.Y.; Zhu, L.F.; Meng, X.J.; Xiao, F.S. Solid acids accelerate the photocatalytic hydrogen peroxide synthesis over a hybrid catalyst of Titania nanotube with carbon dot. Appl. Catal. B Environ. 2019, 244, 594–603. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving efficient H2O2 production by a visible-light absorbing, highly stable photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Duke, F.R.; Haas, T.W. The Homogeneous Base-Catalyzed Decomposition of Hydrogen Peroxide. J. Phys. Chem. 1961, 65, 304–306. [Google Scholar] [CrossRef]
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Garcia-Bastidas, F.; Kema, G.; Waterhouse, P.; Mengersen, K.; et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017. [CrossRef] [Green Version]
- Sunisha, C.; Sowmya, H.D.; Usharani, T.R.; Umesha, M.; Gopalkrishna, H.R.; Saxena, A. Deployment of Stacked Antimicrobial Genes in Banana for Stable Tolerance against Fusarium oxysporum f. sp. cubense through Genetic Transformation. Mol. Biotechnol. 2019, 1–10. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernandez-Ibanez, P.; Polo-Lopez, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2005, 20, 5574–5615. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, T.; Venugopal, G.; Durairaj, A.; Vasanthkumar, S.; Huang, X. Utilization of the internal electric field in semiconductor photocatalysis: A short review. J. Ind. Eng. Chem. 2019, 72, 18–30. [Google Scholar] [CrossRef]
- Wang, F.; Smith, D.W.; El-Din, M.G. Application of advanced oxidation methods for landfill leachate treatment—A review. J. Environ. Eng. Sci. 2003, 2, 413–427. [Google Scholar] [CrossRef]
- Hoshiba, K.; Ponou, J.; Dodbiba, G.; Ito, H.; Sase, T.; Matsui, H.; Fujita, T. Effect of Calcination Temperature on the Hydroxyl Radical Generation of Calcined Dolomite Suspension. J. MMIJ 2018, 134, 151–157. [Google Scholar] [CrossRef]
- Oka, T.; Midoricawa, M.; Saiki, S.; Muroya, Y.; Kamibayasshi, M.; Yamashita, M.; Anzai, K. Spin-Trapping Reactions of a Novel Gauchetype Radical Trapper G-CYPMPO. Anal. Chem. 2011, 83, 9600–9604. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs toward Sustainable Disease Management. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryce, E. Are Bananas Doomed? Live Science, 29 June 2019. Available online: https://www.livescience.com/65830-will-bananas-go-extinct.html (accessed on 1 December 2019).
- Qi, D.F.; Zou, L.P.; Zhou, D.B.; Chen, Y.F.; Gao, Z.F.; Feng, R.J.; Zhang, M.Y.; Li, K.; Wang, W. Taxonomy and Broad-Spectrum Antifungal Activity of Streptomyces sp. SCA3-4 Isolated from Rhizosphere Soil of Opuntia stricta. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Warman, N.M.; Aitken, E.B. The Movement of Fusarium oxysporum f. sp. cubense (Sub-Tropical Race 4) in Susceptible Cultivars of Banana. Front. Plant Sci. 2018. [Google Scholar] [CrossRef] [Green Version]














| Type | As-Received | 800 °C Calcined | |
|---|---|---|---|
| Surface area, (m2/g) | BET | 16.79 | 6.25 |
| Mesopores | 11.65 | 4.68 | |
| Pore Diameter, (nm) | Average | 10.00 | 21.97 |
| Mesopores | 3.38 | 3.06 | |
| Micropores | 0.45 | 0.45 |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, T.; Ponou, J.; Dodbiba, G.; Anh, J.-W.; Lu, S.; Hamza, M.F.; Wei, Y. Removal of Banana Tree Fungi Using Green Tuff Rock Powder Waste Containing Zeolite. Catalysts 2019, 9, 1049. https://doi.org/10.3390/catal9121049
Fujita T, Ponou J, Dodbiba G, Anh J-W, Lu S, Hamza MF, Wei Y. Removal of Banana Tree Fungi Using Green Tuff Rock Powder Waste Containing Zeolite. Catalysts. 2019; 9(12):1049. https://doi.org/10.3390/catal9121049
Chicago/Turabian StyleFujita, Toyohisa, Josiane Ponou, Gjergj Dodbiba, Ji-Whahn Anh, Siminig Lu, Mohammed F. Hamza, and Yuezou Wei. 2019. "Removal of Banana Tree Fungi Using Green Tuff Rock Powder Waste Containing Zeolite" Catalysts 9, no. 12: 1049. https://doi.org/10.3390/catal9121049