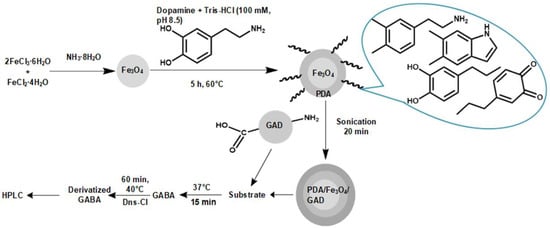
Enhancement of GAD Storage Stability with Immobilization on PDA-Coated Superparamagnetic Magnetite Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fe3O4, Fe3O4/PDA and Fe3O4/PDA/GAD Nanoparticles
2.1.1. X-Ray Diffraction (XRD)
2.1.2. Vibrational Scanning Measurement (VSM)
2.1.3. Thermogravimetric Analysis (TGA)
2.1.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.1.5. Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM)
2.2. Study of Enzyme Activity
2.2.1. High Performance Liquid Chromatography (HPLC)
2.2.2. Activity of the Free and Immobilized Enzyme
2.2.3. Enzyme Reusability
2.2.4. Storage Stability
3. Materials and Methods
3.1. Materials
Bacterial Culture
3.2. Magnetite (Fe3O4) Nanoparticle Synthesis
3.3. Polydopamine Coating of Magnetite Nanoparticles (Fe3O4/PDA)
3.4. Enzyme Expression and Purification
3.5. Enzyme Immobilization
3.6. Characterization of GAD Immobilized on PDA-Coated Fe3O4 Nanoparticles (Fe3O4/PDA/GAD)
3.7. GAD Activity Assay
3.8. High Performance Liquid Chromatography (HPLC) Analysis and Conditions
3.9. Reusability Assay
3.10. Storage Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Q.; Yang, S.; Lü, F.; Lu, Z.; Bie, X.; Jiao, Y.; Zou, X. Cloning and expression of glutamate decarboxylase gene from streptococcus thermophilus y2. J. Gen. Appl. Microbiol. 2009, 55, 305–310. [Google Scholar] [CrossRef]
- Yu, K.; Lin, L.; Hu, S.; Huang, J.; Mei, L. C-terminal truncation of glutamate decarboxylase from lactobacillus brevis cgmcc 1306 extends its activity toward near-neutral pH. Enzyme Microb. Technol. 2012, 50, 263–269. [Google Scholar] [CrossRef]
- Lin, L.; Hu, S.; Yu, K.; Huang, J.; Yao, S.; Lei, Y.; Hu, G.; Mei, L. Enhancing the activity of glutamate decarboxylase from lactobacillus brevis by directed evolution. Chin. J. Chem. Eng. 2014, 22, 1322–1327. [Google Scholar] [CrossRef]
- Enna, S.; McCarson, K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar] [PubMed]
- Nelson, L.; Guo, T.; Lu, J.; Saper, C.; Franks, N.; Maze, M. The sedative component of anesthesia is mediated by GABA a receptors in an endogenous sleep pathway. Nat. Neurosci. 2002, 5, 979. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, K.; Ueno, Y.; Oda, K. Glutamate decarboxylase from lactobacillus brevis: Activation by ammonium sulfate. Biosci. Biotechnol. Biochem. 2008, 72, 1299–1306. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, E.Y.; Noh, W.; Oh, Y.H.; Kim, H.Y.; Song, B.K.; Cho, K.M.; Hong, S.H.; Lee, S.H.; Jegal, J. Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant escherichia coli. Bioprocess Biosyst. Eng. 2013, 36, 885–892. [Google Scholar] [CrossRef]
- Choi, S.-I.; Lee, J.-W.; Park, S.-M.; Lee, M.-Y.; Ji, G.-E.; Park, M.-S.; Heo, T.-R. Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of lactobacillus brevis GABA 057. J. Microbiol. Biotechnol. 2006, 16, 562–568. [Google Scholar]
- Huang, J.; Mei, L.; Wu, H.; Lin, D. Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of lactobacillus brevis. World J. Microbiol. Biotechnol. 2007, 23, 865–871. [Google Scholar] [CrossRef]
- Xu, N.; Wei, L.; Liu, J. Biotechnological advances and perspectives of gamma-aminobutyric acid production. World J. Microbiol. Biotechnol. 2017, 33, 64. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ahn, J.; Lee, J.; Lee, H.; Kim, C.; Jung, J.-K.; Lee, H.; Lee, E.G. Expression, immobilization and enzymatic properties of glutamate decarboxylase fused to a cellulose-binding domain. Int. J. Mol. Sci. 2012, 13, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wu, X.; Zhu, J.; Sun, B.; Miller, C. In vitro enzymatic conversion of γ-aminobutyric acid immobilization of glutamate decarboxylase with bacterial cellulose membrane (BCM) and non-linear model establishment. Enzyme Microb. Technol. 2013, 52, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Poorakbar, E.; Baharifar, H.; Barkhi, M. Recent advances of cellulase immobilization onto magnetic nanoparticles: An update review. Magnetochemistry 2019, 5, 36. [Google Scholar] [CrossRef]
- Mascolo, M.; Pei, Y.; Ring, T. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Zhu, L.-P.; Li, X.-L.; Xu, Y.-Y.; Zhu, B.-K. Surface modification of pe porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Musabirov, I.I.; Sergeev, S.N.; Ivanov, S.P.; Rumyantsev, E.V.; Goncharenko, A. Immobilization of bovine serum albumin onto porous poly(vinylpyrrolidone)-modified silicas. Ind. Eng. Chem. Res. 2014, 53, 13699–13710. [Google Scholar] [CrossRef]
- Martín, M.; Salazar, P.; Jiménez, C.; Lecuona, M.; Ramos, M.J.; Ode, J.; Alcoba, J.; Roche, R.; Villalonga, R.; Campuzano, S. Rapid legionella pneumophila determination based on a disposable core–shell Fe3O4@polydopamine magnetic nanoparticles immunoplatform. Anal. Chim. Acta 2015, 887, 51–58. [Google Scholar] [CrossRef]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, T.; Hu, S.; Xie, D.; Fang, H.; Mei, L.; Huang, J.; Wang, J.; Yao, S. Immobilized glutamate decarboxylase by carboxal magnetic microspheres. J. Chem. Ind. Eng. 2016, 68, 1550–1557. [Google Scholar]
- Veisi, H.; Parvizi, F.; Abdi, M.R. Magnetic solid-phase extraction and uv/vis spectrophotometric determination of trace amount of copper in vegetable and fruit samples after preconcentration of its pentetate complex. J. Nanoanal. 2018, 5, 171–181. [Google Scholar]
- Yu, B.Y.; Kwak, S.-Y. Assembly of magnetite nanocrystals into spherical mesoporous aggregates with a 3-d wormhole-like pore structure. J. Mater.Chem. 2010, 20, 8320. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Zn nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic nanoparticles in medical diagnostic applications: Synthesis, characterization and proteins conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayati, M.; Kakanejadifard, A.; Mohammadi, P.; Abdi, M.R.; Gholami, J.; Hemmati, S. In situ green synthesis of Pd nanoparticles on tannic acid-modified magnetite nanoparticles as a green reductant and stabilizer agent: Its application as a recyclable nanocatalyst (Fe3O4@Ta/Pd) for reduction of 4-nitrophenol and suzuki reactions. ChemistrySelect 2018, 3, 1820–1826. [Google Scholar] [CrossRef]
- An, P.; Zuo, F.; Li, X.; Wu, Y.; Zhang, J.; Zheng, Z.; Ding, X.; Peng, Y. A bio-inspired polydopamine approach to preparation of gold-coated Fe3O4 core–shell nanoparticles: Synthesis, characterization and mechanism. Nano 2013, 8, 1350061. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with polydopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Suárez-García, S.; Sedó, J.; Saiz-Poseu, J.; Ruiz-Molina, D. Copolymerization of a catechol and a diamine as a versatile polydopamine-like platform for surface functionalization: The case of a hydrophobic coating. Biomimetics 2017, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Zhou, Z.; Zhang, R.; Shen, H.; Song, J.; Su, P.; Yang, Y. Enhanced reusability and activity: DNA directed immobilization of enzyme on polydopamine modified magnetic nanoparticles. Biochem. Eng. J. 2018, 137, 108–115. [Google Scholar] [CrossRef]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters affecting the performance of immobilized enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sharma, V.; Majumdar, D.K. Entrapment of alpha-amylase in agar beads for biocatalysis of macromolecular substrate. Int. Sch. Res. Notices 2014, 2014, 936129. [Google Scholar]
- Konwarh, R.; Karak, N.; Rai, S.K.; Mukherjee, A.K. Polymer-assisted iron oxide magnetic nanoparticle immobilized keratinase. Nanotechnology 2009, 20, 225107. [Google Scholar] [CrossRef]
- Dolgormaa, A.; Lv, C.; Li, Y.; Yang, J.; Yang, J.; Chen, P.; Wang, H.; Huang, J. Adsorption of Cu (II) and Zn (II) ions from aqueous solution by gel/PVA-modified super-paramagnetic iron oxide nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Deng, Y.; Lyu, B.; Zhang, R.; Zhang, H.; Ma, H.; Lyu, Y.; Wei, S. Rapidly-deposited polydopamine coating via high temperature and vigorous stirring: Formation, characterization and biofunctional evaluation. PLoS ONE 2014, 9, e113087. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, J.; Lv, C.; Hu, S.; Yao, S.; Mei, L.; Lei, Y. pH stabilization of lactic acid fermentation via the glutamate decarboxylation reaction: Simultaneous production of lactic acid and γ-aminobutyric acid. Process Biochem. 2015, 50, 1523–1527. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, X.; Huang, K.; Xu, H. Modification of Fe3O4 magnetic nanoparticles by L-dopa or dopamine as an enzyme support. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 480–485. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, C.; Liu, L.; Huang, J.; Zhao, W.; Hu, S.; Mei, L.; Yao, S. Biosynthesis of γ-aminobutyrate by engineered lactobacillus brevis cells immobilized in gellan gum gel beads. J. Biosci. Bioeng. 2019, 128, 123–128. [Google Scholar] [CrossRef]








| Time (min) | Flow Rate (mL/min) | A% | B% |
|---|---|---|---|
| 0 | 1 | 20 | 80 |
| 5 | 1 | 20 | 80 |
| 20 | 1 | 50 | 50 |
| 21 | 1 | 100 | 0 |
| 27 | 1 | 100 | 0 |
| 28 | 1 | 20 | 80 |
| 30 | 1 | 20 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, F.; Wang, H.-p.; Lv, C.-j.; Ullah, M.-H.; Liu, C.-y.; Hua, Y.-j.; Mei, L.-h.; Huang, J. Enhancement of GAD Storage Stability with Immobilization on PDA-Coated Superparamagnetic Magnetite Nanoparticles. Catalysts 2019, 9, 969. https://doi.org/10.3390/catal9110969
Zafar F, Wang H-p, Lv C-j, Ullah M-H, Liu C-y, Hua Y-j, Mei L-h, Huang J. Enhancement of GAD Storage Stability with Immobilization on PDA-Coated Superparamagnetic Magnetite Nanoparticles. Catalysts. 2019; 9(11):969. https://doi.org/10.3390/catal9110969
Chicago/Turabian StyleZafar, Farheen, Hong-peng Wang, Chang-jiang Lv, Muhammad-Haseeb Ullah, Chun-yan Liu, Yu-jiao Hua, Le-he Mei, and Jun Huang. 2019. "Enhancement of GAD Storage Stability with Immobilization on PDA-Coated Superparamagnetic Magnetite Nanoparticles" Catalysts 9, no. 11: 969. https://doi.org/10.3390/catal9110969