Ca-Doped CrOX/γ-Al2O3 Catalysts with Improved Dehydrogenation Performance for the Conversion of Isobutane to Isobutene
Abstract
:1. Introduction
2. Results
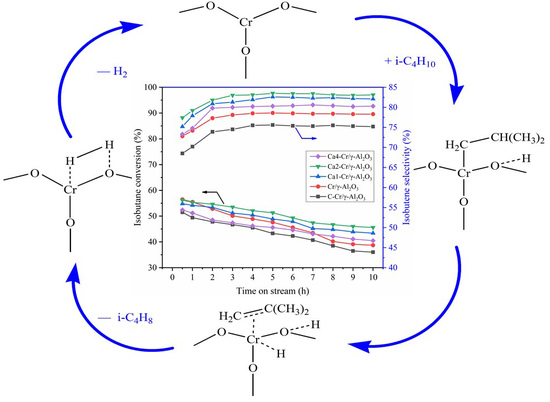
2.1. Isobutane Dehydrogenation Performance
2.2. XRD Analysis
2.3. Textural Properties
2.4. The Analysis of the Acidity Properties
2.5. The Reduction Behavior
2.6. The TG Analysis of the Spent Catalysts
3. Materials and Methods
3.1. The Preparation of Catalysts
3.2. Samples Characterization
3.3. Catalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nawaz, Z. Light alkane dehydrogenation to light olefin technologies: A comprehensive review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar] [CrossRef]
- Ren, Y.; Liao, Z.; Sun, J.; Jiang, B.; Wang, J.; Yang, Y.; Wu, Q. Molecular reconstruction: Recent progress toward composition modeling of petroleum fractions. Chem. Eng. J. 2019, 357, 761–775. [Google Scholar] [CrossRef]
- Symoens, S.H.; Olahova, N.; Gandarillas, A.E.M.; Karimi, H.; Djokic, M.R.; Reyniers, M.F.; Marin, G.B.; Geem, K.M.V. State-of-the-art of coke formation during steam cracking: Anti-coking surface technologies. Ind. Eng. Chem. Res. 2018, 57, 16117–16136. [Google Scholar] [CrossRef]
- Qiao, K.; Peng, P.; Hu, C.; Bai, P.; Yan, Z. Synthesis of vanadium-based catalysts and their excellent catalytic behaviors on dehydrogenation of C4 hydrocarbons. Appl. Petrochem. Res. 2015, 5, 321–327. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Jong, K.P.D. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Shi, J.; Zhang, Y.; Sheng, X.; Zhang, Z. Synthesis of Ce-doped mesoporous γ-alumina with enhanced catalytic performance for propane dehydrogenation. J. Mater. Sci. 2015, 50, 3984–3993. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Merk, A.A.; Salaev, M.A.; Vodyankina, O.V.; Mamontov, G.V. Influence of method of introduction of Cu-and Zn-based modifiers on the properties of chromia–alumina catalysts. Kinet. Catal. 2018, 59, 211–217. [Google Scholar] [CrossRef]
- McFarland, E. Unconventional chemistry for unconventional natural gas. Science 2012, 338, 340–342. [Google Scholar] [CrossRef]
- Sheintuch, M.; Nekhamkina, O. Architecture alternatives for propane dehydrogenation in a membrane reactor. Chem. Eng. J. 2018, 347, 900–912. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A.; Rahimpour, M.R. Optimal conditions of isobutane dehydrogenation in radial flow moving bed hydrogen-permselective membrane reactors to enhance isobutene and hydrogen production. Chem. Eng. Process. 2014, 75, 126–133. [Google Scholar] [CrossRef]
- Ma, Z.; Mo, Y.; Li, J.; An, C.; Liu, X. Optimization of PtSnK/Al2O3 isobutane dehydrogenation catalyst prepared by an impregnation-reduction method. J. Nat. Gas Sci. Eng. 2015, 27, 1035–1042. [Google Scholar] [CrossRef]
- Kilicarslan, S.; Dogan, M.; Dogu, T. Cr Incorporated MCM-41 type catalysts for isobutane dehydrogenation and deactivation mechanism. Ind. Eng. Chem. Res. 2013, 52, 3674–3682. [Google Scholar] [CrossRef]
- Tian, Y.P.; Bai, P.; Liu, S.M.; Liu, X.M.; Yan, Z.F. VOx–K2O/γ-Al2O3 catalyst for nonoxidative dehydrogenation of isobutene. Fuel Process. Technol. 2016, 151, 31–39. [Google Scholar] [CrossRef]
- Wang, G.; Sun, N.; Gao, C.; Zhu, X.; Sun, Y.; Li, C.; Shan, H. Promoting mechanism of sulfur addition in catalytic dehydrogenation of isobutane over Mo/MgAl2O4 catalysts. Appl. Catal. A Gen. 2014, 478, 71–80. [Google Scholar] [CrossRef]
- Zhao, H.; Song, H.; Chou, L. Synthesis and catalytic application in isobutane dehydrogenation of the mesoporous chromia/alumina catalysts based on a metal–organic framework. Microporous Mesoporous Mater. 2013, 181, 182–191. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Mamontov, G.V. The Study of CrOx-containing catalysts supported on ZrO2, CeO2, and CexZr(1–x)O2 in isobutane dehydrogenation. Kinet. Catal. 2018, 59, 143–149. [Google Scholar] [CrossRef]
- Matveyeva, A.N.; Zaitseva, N.; Mäki-Arvela, P.; Aho, A.; Bachina, A.; Fedorov, S.P.; Murzin, D.Y.; Pakhomov, N. Fluidized-bed isobutane dehydrogenation over alumina-supported Ga2O3 and Ga2O3–Cr2O3 catalysts. Ind. Eng. Chem. Res. 2018, 57, 927–938. [Google Scholar] [CrossRef]
- Li, P.P.; Lang, W.Z.; Xia, K.; Luan, L.; Yan, X.; Guo, Y.J. The promotion effects of Ni on the properties of Cr/Al catalysts for propane dehydrogenation reaction. Appl. Catal. A Gen. 2016, 522, 172–179. [Google Scholar] [CrossRef]
- Cavani, F.; Koutyrev, M.; Trifiro, F.; Bartolini, A.; Ghisletti, D.; Iezzi, R.; Piero, G.D. Chemical and physical characterization of alumina-supported chromia-based catalysts and their activity in dehydrogenation of isobutane. J. Catal. 1996, 158, 236–250. [Google Scholar] [CrossRef]
- Neri, G.; Pistone, A.; Rossi, S.D.; Rombi, E.; Milone, C.; Galvagno, S. Ca-doped chromium oxide catalysts supported on alumina for the oxidative dehydrogenation of isobutene. Appl. Catal. A Gen. 2004, 260, 75–86. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Airaksinen, S.M.K.; Bañares, M.A.; Krause, A.O.I. Isobutane dehydrogenation on zirconia-, alumina-, and zirconia/alumina-supported chromia catalysts. Appl. Catal. A Gen. 2007, 333, 30–41. [Google Scholar] [CrossRef]
- Martyanov, I.; Sayari, A. Sol–gel assisted preparation of chromia–silica catalysts for non-oxidative dehydrogenation of propane. Catal. Lett. 2008, 126, 164–172. [Google Scholar] [CrossRef]
- Tsyganok, A.; Green, R.G.; Giorgi, J.B.; Sayari, A. Non-oxidative dehydrogenation of ethane to ethylene over chromium catalysts prepared from layered double hydroxide precursors. Catal. Commun. 2007, 8, 2186–2193. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Song, H.; Chou, L. Catalytic dehydrogenation of isobutane over ordered mesoporous Cr2O3–Al2O3 composite oxides. Catal. Commun. 2013, 35, 76–81. [Google Scholar] [CrossRef]
- Bai, Y.K.; Zheng, R.T.; Gu, Q.; Wang, J.J.; Wang, B.S.; Cheng, G.A.; Chen, G. One-step synthesis of hollow Cr(OH)3 micro/nano-hexagonal pellets and the catalytic properties of hollow Cr2O3 structures. J. Mater. Chem. A 2014, 2, 12770–12775. [Google Scholar] [CrossRef]
- Shee, D.; Sayari, A. Light alkane dehydrogenation over mesoporous Cr2O3/Al2O3 catalysts. Appl. Catal. A Gen. 2010, 389, 155–164. [Google Scholar] [CrossRef]
- Tom, W.D.; Carlos, H.M.; Krijn, P.J. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar]
- Tigran, M.; Kim, L.; Sung, M.K.; Frank, K.; Alexey, F.; Peter, C.; Christoph, R.M.; Christophe, C. Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar]
- Jonathan, H.; Stephanie, L.; Rose, A.; Jason, S. The Impact of La Doping on Dry Reforming Ni-Based Catalysts Loaded on FSP-Alumina. Top. Catal. 2018, 61, 1842–1855. [Google Scholar]
- Guangjian, W.; Xingyuan, S.; Xinshan, N.; Fanfei, M.; Fang, W. Effect of chelating agents on catalytic performance of Cr/γ-Al2O3 dehydrogenation catalysts. Chem. Pap. 2018, 72, 921–928. [Google Scholar]
- Han, D.; Li, X.; Zhang, L.; Wang, Y.; Yan, Z.; Liu, S. Hierarchically ordered meso/macroporous γ-alumina for enhanced hydrodesulfurization performance. Microporous Mesoporous Mater. 2012, 158, 1–6. [Google Scholar] [CrossRef]
- Xing, T.; Wan, H.; Shao, Y.; Han, Y.; Xu, Z.; Zheng, S. Catalytic combustion of benzene over γ-alumina supported chromium oxide catalysts. Appl. Catal. A Gen. 2013, 468, 269–275. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, H.; Yang, J.; Zhao, J.; Yan, L.; Song, H.; Chou, L.J. The catalytic dehydrogenation of isobutane and the stability enhancement over Fe incorporated SBA-15. Microporous Mesoporous Mater. 2018, 266, 117–125. [Google Scholar] [CrossRef]
- Lai, Y.; He, S.; Li, X.; Sun, C.; Seshan, K. Dehydrogenation of n-dodecane over PtSn/MgAlO catalysts: Investigating the catalyst performance while monitoring the products. Appl. Catal. A Gen. 2014, 469, 74–80. [Google Scholar] [CrossRef]
- Narayanan, S.; Sultana, A.; Le, Q.T.; Auroux, A. A comparative and multitechnical approach to the acid character of templated and non-templated ZSM-5 zeolites. Appl. Catal. A Gen. 1998, 168, 373–384. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: Insight into the nature of catalytically active chromium sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Zhao, H.; Song, H.; Xu, L.; Chou, L. Isobutane dehydrogenation over the mesoporous Cr2O3/Al2O3 catalysts synthesized from a metal-organic framework MIL-101. Appl. Catal. A Gen. 2013, 456, 188–196. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Xing, R.; Severance, M. Investigating the CrOx/Al2O3 dehydrogenation catalyst model: I. identification and stability evaluation of the Cr species on the fresh and equilibrated catalysts. Appl. Catal. A Gen. 2016, 523, 39–53. [Google Scholar] [CrossRef]






| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) |
|---|---|---|
| γ-Al2O3 | 265 | 0.43 |
| C–Cr/γ-Al2O3 | 127 | 0.35 |
| Cr/γ-Al2O3 | 221 | 0.41 |
| Ca1–Cr/γ-Al2O3 | 213 | 0.40 |
| Ca2–Cr/γ-Al2O3 | 210 | 0.39 |
| Ca4–Cr/γ-Al2O3 | 203 | 0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Song, N.; Lu, K.; Wang, W.; Bing, L.; Zhang, Q.; Fu, H.; Wang, F.; Han, D. Ca-Doped CrOX/γ-Al2O3 Catalysts with Improved Dehydrogenation Performance for the Conversion of Isobutane to Isobutene. Catalysts 2019, 9, 968. https://doi.org/10.3390/catal9110968
Wang G, Song N, Lu K, Wang W, Bing L, Zhang Q, Fu H, Wang F, Han D. Ca-Doped CrOX/γ-Al2O3 Catalysts with Improved Dehydrogenation Performance for the Conversion of Isobutane to Isobutene. Catalysts. 2019; 9(11):968. https://doi.org/10.3390/catal9110968
Chicago/Turabian StyleWang, Guangjian, Ning Song, Kai Lu, Wentai Wang, Liancheng Bing, Qinqin Zhang, Haitao Fu, Fang Wang, and Dezhi Han. 2019. "Ca-Doped CrOX/γ-Al2O3 Catalysts with Improved Dehydrogenation Performance for the Conversion of Isobutane to Isobutene" Catalysts 9, no. 11: 968. https://doi.org/10.3390/catal9110968