Alkali-Free Zn–Al Layered Double Hydroxide Catalysts for Triglyceride Transesterification
Abstract
:1. Introduction
2. Results and Discussion
2.1. ZnAl-LDH Synthesis and Characterization
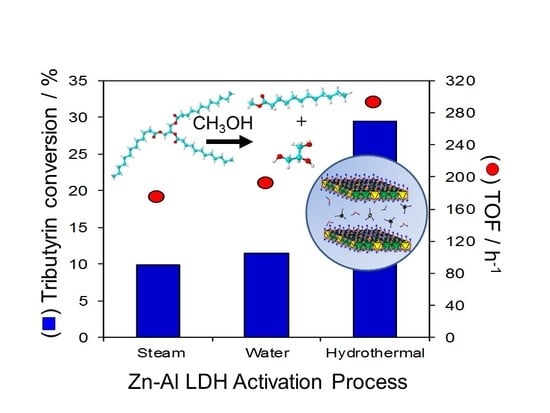
2.2. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterisation
3.3. Catalytic Transesterification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M.M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Ma, F.; Clements, L.D.; Hanna, M.A. Biodiesel Fuel from Animal Fat. Ancillary Studies on Transesterification of Beef Tallow. Ind. Eng. Chem. Res. 1998, 37, 3768–3771. [Google Scholar] [CrossRef]
- Mandolesi de Araújo, C.D.; de Andrade, C.C.; de Souza e Silva, E.; Dupas, F.A. Biodiesel production from used cooking oil: A review. Renew. Sustain. Energy Rev. 2013, 27, 445–452. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel: Current Trends and Properties. Top. Catal. 2010, 53, 714–720. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Narasimharao, K.; Lee, A.; Wilson, K. Catalysts in Production of Biodiesel: A Review. J. Biobased Mater. Bioenergy 2007, 1, 19–30. [Google Scholar] [CrossRef]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as feasible petrol fuel replacement: A multidisciplinary overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Ono, Y.; Baba, T. Selective reactions over solid base catalysts. Catal. Today 1997, 38, 321–337. [Google Scholar] [CrossRef]
- Dacquin, J.-P.; Lee, A.F.; Wilson, K. Chapter 16 Heterogeneous Catalysts for Biodiesel Production. In Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; The Royal Society of Chemistry: London, UK, 2010; pp. 416–434. [Google Scholar]
- Wilson, K.; Lee, A.F. Rational design of heterogeneous catalysts for biodiesel synthesis. Catal. Sci. Technol. 2012, 2, 884. [Google Scholar] [CrossRef]
- Ramachandran, K.; Suganya, T.; Nagendra Gandhi, N.; Renganathan, S. Recent developments for biodiesel production by ultrasonic assist transesterification using different heterogeneous catalyst: A review. Renew. Sustain. Energy Rev. 2013, 22, 410–418. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Pirez, C.; Harvey, A.P.; Lee, A.F.; Wilson, K. Heterogeneous catalysis in an oscillatory baffled flow reactor. Catal. Sci. Technol. 2013, 3, 2373–2379. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Othman, M.R.; Helwani, Z.; Martunus; Fernando, W.J.N. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: A review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New synthetic routes to hydrotalcite-like compounds—Characterisation and properties of the obtained materials. Eur. J. Inorg. Chem. 1998, 1439–1446. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, N.; Mayoral, J.A.; Pires, E.; Roldán, L. The influence of alkaline metals on the strong basicity of Mg–Al mixed oxides: The case of transesterification reactions. Appl. Catal. A Gen. 2009, 364, 87–94. [Google Scholar] [CrossRef]
- Cross, H.E.; Brown, D.R. Entrained sodium in mixed metal oxide catalysts derived from layered double hydroxides. Catal. Commun. 2010, 12, 243–245. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Woodford, J.J.; Dacquin, J.-P.; Wilson, K.; Lee, A.F. Better by design: Nanoengineered macroporous hydrotalcites for enhanced catalytic biodiesel production. Energy Environ. Sci. 2012, 5, 6145–6150. [Google Scholar] [CrossRef]
- Helwani, Z.; Aziz, N.; Kim, J.; Othman, M.R. Improving the yield of Jatropha curcas’s FAME through sol–gel derived meso-porous hydrotalcites. Renew. Energy 2016, 86, 68–74. [Google Scholar] [CrossRef]
- Hibino, T.; Ohya, H. Synthesis of crystalline layered double hydroxides: Precipitation by using urea hydrolysis and subsequent hydrothermal reactions in aqueous solutions. Appl. Clay Sci. 2009, 45, 123–132. [Google Scholar] [CrossRef]
- Montanari, T.; Sisani, M.; Nocchetti, M.; Vivani, R.; Delgado, M.C.H.; Ramis, G.; Busca, G.; Costantino, U. Zinc–aluminum hydrotalcites as precursors of basic catalysts: Preparation, characterization and study of the activation of methanol. Catal. Today 2010, 152, 104–109. [Google Scholar] [CrossRef]
- Quirino, M.R.; Oliveira, M.J.C.; Keyson, D.; Lucena, G.L.; Oliveira, J.B.L.; Gama, L. Synthesis of zinc aluminate with high surface area by microwave hydrothermal method applied in the transesterification of soybean oil (biodiesel). Mater. Res. Bull. 2016, 74, 124–128. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, H.-F.; Qi, T.; Yan, S.-L.; Liang, B. Preparation, application, and optimization of Zn/Al complex oxides for biodiesel production under sub-critical conditions. Biotechnol. Adv. 2010, 28, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.H.G.; Rouxel, J.-J.; Leporq, S. Process for the Production of Esters from Vegetable oils or Animal Oils Alcohols. U.S. Patent 5,908,946, 1 June 1999. [Google Scholar]
- Liu, Q.; Wang, B.; Wang, C.; Tian, Z.; Qu, W.; Ma, H.; Xu, R. Basicities and transesterification activities of Zn–Al hydrotalcites-derived solid bases. Green Chem. 2014, 16, 2604–2613. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Groen, J.C.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol Condensations Over Reconstructed Mg–Al Hydrotalcites: Structure–Activity Relationships Related to the Rehydration Method. Chem. A Eur. J. 2004, 11, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Livi, K.J.T.; Xu, W.; Siebecker, M.G.; Wang, Y.; Phillips, B.L.; Sparks, D.L. Formation of Crystalline Zn–Al Layered Double Hydroxide Precipitates on γ-Alumina: The Role of Mineral Dissolution. Environ. Sci. Technol. 2012, 46, 11670–11677. [Google Scholar] [CrossRef] [PubMed]
- Prikhod’ko, R.V.; Sychev, M.V.; Astrelin, I.M.; Erdmann, K.; Mangel, A.; van Santen, R.A. Synthesis and Structural Transformations of Hydrotalcite-like Materials Mg-Al and Zn-Al. Russ. J. Appl. Chem. 2001, 74, 1621–1626. [Google Scholar] [CrossRef]
- Kooli, F.; Depege, C.; Ennaqadi, A.; de Roy, A.; Besse, J.P. Rehydration of Zn-Al layered double hydroxides. Clays Clay Miner. 1997, 45, 92–98. [Google Scholar] [CrossRef]
- Pachayappan, L.; Nagendran, S.; Kamath, P.V. Reappraisal of Polytypism in Layered Double Hydroxides: Consequences of Cation Ordering in the Metal Hydroxide Layer. Cryst. Growth Des. 2017, 17, 2536–2543. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I.; O’Hare, D. Time-resolved in situ X-ray diffraction study of the liquid-phase reconstruction of Mg–Al–carbonate hydrotalcite-like compounds. J. Mater. Chem. 2000, 10, 1713–1720. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effects of synthesis pH and hydrothermal treatment on the formation of zinc aluminum hydrotalcites. J. Solid State Chem. 2004, 177, 4047–4057. [Google Scholar] [CrossRef] [Green Version]
- Boclair, J.W.; Braterman, P.S. Layered Double Hydroxide Stability. 1. Relative Stabilities of Layered Double Hydroxides and Their Simple Counterparts. Chem. Mater. 1999, 11, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. From Layered Double Hydroxides to ZnO-based Mixed Metal Oxides by Thermal Decomposition: Transformation Mechanism and UV-Blocking Properties of the Product. Chem. Mater. 2010, 22, 3933–3942. [Google Scholar] [CrossRef]
- Tichit, D.; Bennani, M.N.; Figueras, F.; Tessier, R.; Kervennal, J. Aldol condensation of acetone over layered double hydroxides of the meixnerite type. Appl. Clay Sci. 1998, 13, 401–415. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Perez-Ramirez, J.; Cesteros, Y.; Salagre, P.; Sueiras, J. Nanoplatelet-based reconstructed hydrotalcites: Towards more efficient solid base catalysts in aldol condensations. Chem. Commun. 2005, 1453–1455. [Google Scholar] [CrossRef] [PubMed]
- Creasey, J.J.; Chieregato, A.; Manayil, J.C.; Parlett, C.M.A.; Wilson, K.; Lee, A.F. Alkali- and nitrate-free synthesis of highly active Mg–Al hydrotalcite-coated alumina for FAME production. Catal. Sci. Technol. 2014, 4, 861–870. [Google Scholar] [CrossRef]
- Montero, J.M.; Brown, D.R.; Gai, P.L.; Lee, A.F.; Wilson, K. In situ studies of structure-reactivity relations in biodiesel synthesis over nanocrystalline MgO. Chem. Eng. J. 2010, 161, 332–339. [Google Scholar] [CrossRef]
- Rabee, A.; Manayil, J.; Isaacs, M.; Parlett, C.; Durndell, L.; Zaki, M.; Lee, A.; Wilson, K. On the Impact of the Preparation Method on the Surface Basicity of Mg–Zr Mixed Oxide Catalysts for Tributyrin Transesterification. Catalysts 2018, 8, 228. [Google Scholar] [CrossRef]
- Campbell, C.T.; Sellers, J.R.V. Enthalpies and Entropies of Adsorption on Well-Defined Oxide Surfaces: Experimental Measurements. Chem. Rev. 2013, 113, 4106–4135. [Google Scholar] [CrossRef] [PubMed]







| Nominal Zn:Al Atomic Ratio | Bulk Composition a | Experimental Zn:Al Atomic Ratio a | BET b Surface Area/m2·g−1 | Crystallite c Size/nm | |
|---|---|---|---|---|---|
| Zn/wt% | Al/wt% | ||||
| 1.5 | 30.9 | 8.0 | 1.6 | 43 | 18 |
| 2.0 | 32.3 | 6.7 | 2.0 | 63 | 15 |
| 3.0 | 33.5 | 4.6 | 3.0 | 73 | 18 |
| 4.0 | 44.2 | 5.5 | 3.3 | 65 | 13 |
| Reconstruction Protocol | LDH Crystallite Size a/nm | %Zn–Al-LDH b | BET Surface Area c/m2·g−1 | Base Site Loading d | |
|---|---|---|---|---|---|
| molecules·g−1 | mmol·g−1 | ||||
| None | - | 0 | 41 | 1.4 × 1019 | 0.023 |
| Steam | 15 | 64 | 81 | 2.4 × 1019 | 0.041 |
| Water | 25 | 64 | 87 | 2.6 × 1019 | 0.044 |
| Hydrothermal | 27 | 73 | 53 | 6.0 × 1019 | 0.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajuddin, N.A.; Manayil, J.C.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Alkali-Free Zn–Al Layered Double Hydroxide Catalysts for Triglyceride Transesterification. Catalysts 2018, 8, 667. https://doi.org/10.3390/catal8120667
Tajuddin NA, Manayil JC, Isaacs MA, Parlett CMA, Lee AF, Wilson K. Alkali-Free Zn–Al Layered Double Hydroxide Catalysts for Triglyceride Transesterification. Catalysts. 2018; 8(12):667. https://doi.org/10.3390/catal8120667
Chicago/Turabian StyleTajuddin, Nazrizawati A., Jinesh C. Manayil, Mark A. Isaacs, Christopher M.A. Parlett, Adam F. Lee, and Karen Wilson. 2018. "Alkali-Free Zn–Al Layered Double Hydroxide Catalysts for Triglyceride Transesterification" Catalysts 8, no. 12: 667. https://doi.org/10.3390/catal8120667