Comparing the Efficiency of N-Doped TiO2 and N-Doped Bi2MoO6 Photo Catalysts for MB and Lignin Photodegradation
Abstract
:1. Introduction
2. Results
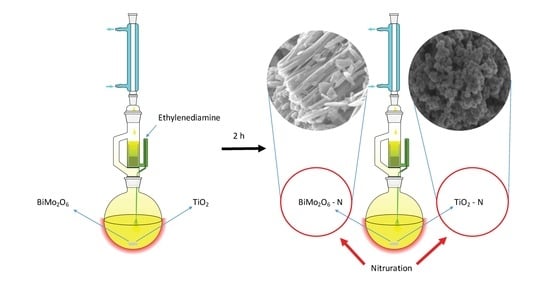
2.1. Scanning Electron Microscopy (SEM)
2.2. X-ray Diffraction Analysis
2.3. Specific Surface Area Determination
2.4. Diffuse Reflectance Measurements
2.5. X-ray Photoelectron Spectroscopy (XPS)
3. Photocatalytic Activity Tests
4. Methods and Materials
4.1. Synthesis of TiO2 and Bi2MoO6 Catalysts
4.2. Synthesis of N-doped TiO2 and N-doped Bi2MoO6
4.3. Characterization Details
4.4. Photocatalytic Evaluation
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical over-view of processes for removing organic dyes from wastewater effluents. Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring Surface–Bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, F.; Wu, T.; Wu, Q.; Huang, Z.; Su, H.; Zhang, Z. Photochemical preparation of CdS hollow microspheres at room temperature and their use in visible-light photocatalysis. J. Solid State Chem. 2011, 184, 644–648. [Google Scholar] [CrossRef]
- Abdelkadera, E.; Nadjia, L.; Naceur, B.; Noureddine, B. SnO2 foam grain-shaped nanoparticles: Synthesis, characterization and UVA light induced photocatalysis. J. Alloys Compd. 2016, 679, 408–419. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Ullaha, S.; Ferreira-Neto, E.P.; Hazra, C.; Parveen, R.; Rojas-Mantilla, H.D.; Calegaro, M.L.; Serge-Correales, Y.E.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Broad spectrum photocatalytic system based on BiVO4 and NaYbF4:Tm3+ upconversion particles for environmental remediation under UV-vis-NIR illumination. Appl. Catal. B Environ. 2019, 243, 121–135. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 nanoparticles obtained by a facile coprecipitation method at low temperature. Ceram. Int. 2018, 44, 5273–5283. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Kontos, A.G.; Likodimos, V.; Katsaros, F.; Boukos, N.; Tsoutsou, D.; Dimoulas, A.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Inorganic-organic core-shell titania nanoparticles for efficient visible light activated photocatalysis. Appl. Catal. B Environ. 2013, 130–131, 14–24. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Patience, G.S.; Boffito, D.C. Spray dried TiO2/WO3 heterostructure for photocatalytic applications with residual activity in the dark. Appl. Catal. B Environ. 2018, 226, 311–323. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Cat. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Rangel, R.; Cedeño, V.; Ramos-Corona, A.; Gutierrez, R.; Alvarado-Gil, J.J.; Ares, O.; Bartolo-Perez, P.; Quintana, P. Tailoring surface and photocatalytic properties of ZnO and nitrogen-doped ZnO nanostructures using microwave-assisted facile hydrothermal synthesis. Appl. Phys. 2017, 123, 552. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Synthesis and characterization of visible light-driven W-doped Bi2MoO6 photocatalyst and its photocatalytic activities. Mater. Lett. 2017, 194, 114–117. [Google Scholar] [CrossRef]
- Rangel, R.; Maya, R.; García, R. Novel [Ce1−XLaxO2, La2−yCeyO3]/Bi2Mo0.9W0.1O6 Catalysts for CO Oxidation at low temperature. Catal. Sci. Technol. 2012, 2, 639–642. [Google Scholar] [CrossRef]
- Geng, B.; Wei, B.; Gao, H.; Xu, L. Ag2O nanoparticles decorated hierarchical Bi2MoO6 microspheres for efficient visible light photocatalysts. J. Alloys Compd. 2017, 699, 783–787. [Google Scholar] [CrossRef]
- Guo, J.; Shi, L.; Zhao, J.; Wang, Y.; Yuan, X. Enhanced visible-light photocatalytic activity of Bi2MoO6 nanoplates with heterogeneous Bi2MoO6-x and Bi2MoO6 core-shell structure. App. Catal. B Environ. 2018, 224, 692–704. [Google Scholar] [CrossRef]
- Umapathy, V.; Manikandan, A.; Antony, S.A.; Ramu, P.; Neeraja, P. Structure, morphology and opto-magnetic properties of Bi2MoO6 nano-photocatalyst synthesized by sol-gel method. Trans. Nonferrous Metals Soc. China 2015, 25, 3271–3278. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Simple solvothermal routes to synthesize nanocrystalline Bi2MoO6 photocatalysts with different morphologies. Acta Mater. 2007, 55, 4699–4705. [Google Scholar] [CrossRef]
- Martínez-de la Cruz, A.; Obregón Alfaro, S. Synthesis and characterization of γ-Bi2MoO6 prepared by co-precipitation: Photoassisted degradation of organic dyes under vis-irradiation. J. Mol. Catal. A Chem. 2010, 320, 85–91. [Google Scholar] [CrossRef]
- Jin, S.; Hao, H.; Gan, Y.; Guo, W.; Li, H.; Hu, X.; Hou, H.; Zhang, G.; Yan, S.; Gao, W.; et al. Preparation and improved photocatalytic activities of Ho3+/Yb3+ co-doped Bi2MoO6. Mater. Chem. Phys. 2017, 199, 107–112. [Google Scholar] [CrossRef]
- Yu, Ch.; Wu, Z.; Liu, R.; Dionysiou, D.D.; Yang, K.; Wang, Ch.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. App. Catal. B Environ. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Xing, Y.; Gao, X.; Ji, G.; Liu, Z.; Du, C. Synthesis of carbon doped Bi2MoO6 for enhanced photocatalytic performance and tumor photodynamic therapy efficiency. Appl. Surf. Sci. 2019, 465, 369–382. [Google Scholar] [CrossRef]
- Asashi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible light photocatalysis in nitrogen-doped titanium oxide. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tafen, D.; Lewis, J.; Hong, Z.; Manivannan, A.; Zhi, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Niimi, S.; Yamamoto, M.; Ogawa, S.; Nomoto, T.; Yagi, S. Characterization of nitrogen ion implanted TiO2 photocatalysts by XAFS and XPS. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2015, 365, 79–81. [Google Scholar] [CrossRef]
- Manova, D.; Franco-Arias, L.; Hofele, A.; Alani, I.; Kleiman, A.; Asenova, I.; Decker, U.; Marquez, A.; Mändl, S. Nitrogen incorporation during PVD deposition of TiO2:N thin films. Surf. Coat. Technol. 2017, 312, 61–65. [Google Scholar] [CrossRef]
- Boningaria, T.; Reddy-Inturia, S.N.; Suidan, D.; Smirniotisa, P.G. Novel one-step synthesis of nitrogen-doped TiO2 by flame aerosol technique T for visible-light photocatalysis: Effect of synthesis parameters and secondary nitrogen (N) source. Chem. Eng. J. 2018, 350, 324–334. [Google Scholar] [CrossRef]
- Tran, V.A.; Truong, T.T.; Pham-Phan, T.A.; Nguyen, T.N.; Huynh, T.V.; Agresti, A.; Pescetelli, S.; Le, T.K.; Carlo, A.; Lund, T.; et al. Application of nitrogen-doped TiO2 nano-tubes in dye-sensitized solar cells. Appl. Surf. Sci. 2017, 399, 515–522. [Google Scholar] [CrossRef]
- Rangel, R.; López-Mercado, G.J.; Bartolo-Pérez, P.; García, R. Nanostructured-[CeO2, La2O3, C]/TiO2 catalysts for lignin photodegradation. Sci. Adv. Mater. 2012, 4, 573–578. [Google Scholar] [CrossRef]
- Rangel, R.; García-Espinoza, J.D.; Espitia-Cabrera, I.; Alvarado-Gil, J.J.; Quintana, P.; Bartolo-Pérez, P.; Trejo-Tzab, R. Synthesis of Mesoporous of NyTi1-xCexO2-y Structures and its Visible Light Induced Photocatalytic Performance. Nano 2013, 8, 1350051–1350061. [Google Scholar] [CrossRef]
- Dahm, A.; Lucian, A. Titanium dioxide catalyzed photodegradation of lignin in industrial effluents. Ind. Eng. Chem. Res. 2004, 43, 7996–8000. [Google Scholar] [CrossRef]
- Gazi, S.; Hung Ng, W.K.; Ganguly, R.; Putra, A.M.; Hirao, H.; Soo, H.S. Selective photocatalytic C–C bond cleavage under ambient conditions with earth abundant vanadium complexes. Chem. Sci. 2015, 6, 7130–7142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Mer, V.K; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. Ind. Eng. Chem. 1950, 72, 4847–4854. [Google Scholar]
- Wnek, W.J. The simulation of precipitation kinetics. Powder Technol. 1978, 20, 289–293. [Google Scholar] [CrossRef]









| Compound | BET Surface Area (m2/g) |
|---|---|
| TiO2 | 117.0 |
| N-TiO2 | 73.7 |
| Bi2MoO6 | 3.8 |
| N-Bi2MoO6 | 2.0 |
| Compound | Experimental Gap, eV | Reported Gap, eV |
|---|---|---|
| TiO2 | 3.17 | 3.20 |
| N-TiO2 | 2.96 | |
| Bi2MoO6 | 2.84 | 2.90 |
| N-Bi2MoO6 | 2.73 |
| Compound | Ti2p | O1s | N1s | Bi | Mo |
|---|---|---|---|---|---|
| TiO2 | 22.73 | 67.77 | - | - | - |
| N-TiO2 | 27.01 | 67.05 | 5.94 | - | - |
| Bi2MoO6 | - | 61.86 | - | 24.12 | 14.02 |
| N-Bi2MoO6 | - | 52.3 | 13.38 | 25.29 | 9.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, R.; Cedeño, V.J.; Espino, J.; Bartolo-Pérez, P.; Rodríguez-Gattorno, G.; Alvarado-Gil, J.J. Comparing the Efficiency of N-Doped TiO2 and N-Doped Bi2MoO6 Photo Catalysts for MB and Lignin Photodegradation. Catalysts 2018, 8, 668. https://doi.org/10.3390/catal8120668
Rangel R, Cedeño VJ, Espino J, Bartolo-Pérez P, Rodríguez-Gattorno G, Alvarado-Gil JJ. Comparing the Efficiency of N-Doped TiO2 and N-Doped Bi2MoO6 Photo Catalysts for MB and Lignin Photodegradation. Catalysts. 2018; 8(12):668. https://doi.org/10.3390/catal8120668
Chicago/Turabian StyleRangel, Ricardo, Verónica Janneth Cedeño, Jaime Espino, Pascual Bartolo-Pérez, Geonel Rodríguez-Gattorno, and Juan José Alvarado-Gil. 2018. "Comparing the Efficiency of N-Doped TiO2 and N-Doped Bi2MoO6 Photo Catalysts for MB and Lignin Photodegradation" Catalysts 8, no. 12: 668. https://doi.org/10.3390/catal8120668