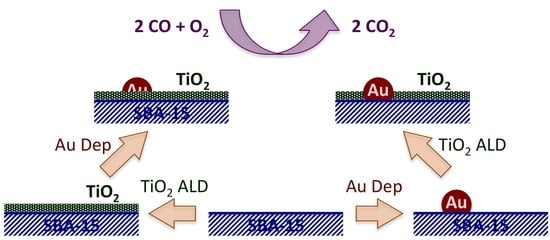
CO Oxidation Catalyzed by Au Dispersed on SBA-15 Modified with TiO2 Films Grown via Atomic Layer Deposition (ALD)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cavani, F. Catalytic selective oxidation: The forefront in the challenge for a more sustainable chemical industry. Catal. Today 2010, 157, 8–15. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J. Industrial Catalysis: A Practical Approach, 3rd ed.; Wiley: Weinheim, Germany, 2015; p. 544. [Google Scholar]
- Védrine, J.C.; Fechete, I. Heterogeneous partial oxidation catalysis on metal oxides. Comptes Rendus Chim. 2016, 19, 1203–1225. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Haruta, M. A golden age of catalysis: A perspective. Appl. Catal. A 2005, 291, 2–5. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press; World Scientific Publishing: London, UK, 2006; p. 384. [Google Scholar]
- Min, B.K.; Friend, C.M. Heterogeneous gold-based catalysis for green chemistry: Low-temperature CO oxidation and propene oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Lee, I.; Joo, J.B.; Yin, Y.; Zaera, F. A Yolk@Shell Nanoarchitecture for Au/TiO2 Catalysts. Angew. Chem. Int. Ed. 2011, 50, 10208–10211. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016, 71, 77–271. [Google Scholar] [CrossRef]
- Zaera, F. Gold-Titania Catalysts for Low-Temperature Oxidation and Water Splitting. Top. Catal. 2018, 61, 336–347. [Google Scholar] [CrossRef]
- Lee, I.; Zaera, F. Catalytic oxidation of carbon monoxide at cryogenic temperatures. J. Catal. 2014, 319, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Joo, J.B.; Yin, Y.; Zaera, F. Au@Void@TiO2 yolk–shell nanostructures as catalysts for the promotion of oxidation reactions at cryogenic temperatures. Surf. Sci. 2016, 648, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Zaera, F. Effect of metal nanoparticle size and titania crystallinity on the performance of Au/TiO2 catalysts for the promotion of carbon monoxide oxidation at cryogenic temperatures. J. Chem. Phys. 2019, 151, 054701. [Google Scholar] [CrossRef]
- Lee, I.; Zaera, F. Use of Au@Void@TiO2 yolk-shell nanostructures to probe the influence of oxide crystallinity on catalytic activity for low-temperature oxidations. J. Chem. Phys. 2019, 151, 234706. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T., Jr. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.; Zhang, T.; Mou, C.-Y. Catalysis by gold: New insights into the support effect. Nano Today 2013, 8, 403–416. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Yang, F.; Evans, J.; Rodriguez, J.A.; Liu, P. CO Oxidation on Gold-Supported Iron Oxides: New Insights into Strong Oxide–Metal Interactions. J. Phys. Chem. C 2015, 119, 16614–16622. [Google Scholar] [CrossRef]
- Odarchenko, Y.; Martin, D.J.; Arnold, T.; Beale, A.M. CO oxidation over supported gold nanoparticles as revealed by operando grazing incidence X-ray scattering analysis. Faraday Discuss. 2018, 208, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz, C.P.; Navarro-Jaén, S.; Rossi, L.M.; Dumeignil, F.; Ghazzal, M.N.; Wojcieszak, R. Enhancing the activity of gold supported catalysts by oxide coating: Towards efficient oxidations. Green Chem. 2021, 23, 8453–8457. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.A.; Yang, N.; Bent, S.F. Nanoengineering Heterogeneous Catalysts by Atomic Layer Deposition. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 41–62. [Google Scholar] [CrossRef]
- Onn, T.M.; Küngas, R.; Fornasiero, P.; Huang, K.; Gorte, R.J. Atomic Layer Deposition on Porous Materials: Problems with Conventional Approaches to Catalyst and Fuel Cell Electrode Preparation. Inorganics 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Zaera, F. Molecular approaches to heterogeneous catalysis. Coord. Chem. Rev. 2021, 448, 214179. [Google Scholar] [CrossRef]
- Zaera, F. Designing Sites in Heterogeneous Catalysis: Are We Reaching Selectivities Competitive with Those of Homogeneous Catalysts? Chem. Rev. 2022, 122, 8594–8757. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Chen, Z.-h.; Qin, X.; Zaera, F. Sub-monolayer control of the growth of oxide films on mesoporous materials. J. Mater. Chem. A 2018, 6, 17548–17558. [Google Scholar] [CrossRef]
- Weng, Z.; Zaera, F. Sub-Monolayer Control of Mixed-Oxide Support Composition in Catalysts via Atomic Layer Deposition: Selective Hydrogenation of Cinnamaldehyde Promoted by (SiO2-ALD)-Pt/Al2O3. ACS Catal. 2018, 8, 8513–8524. [Google Scholar] [CrossRef]
- Weng, Z.; Zaera, F. Atomic Layer Deposition (ALD) as a Way to Prepare New Mixed-Oxide Catalyst Supports: The Case of Alumina Addition to Silica-Supported Platinum for the Selective Hydrogenation of Cinnamaldehyde. Top. Catal. 2019, 62, 838–848. [Google Scholar] [CrossRef]
- Ke, W.; Qin, X.; Palomino, R.M.; Simonovis, J.P.; Senanayake, S.D.; Rodriguez, J.A.; Zaera, F. Redox Properties of TiO2 Thin Films Grown on Mesoporous Silica by Atomic Layer Deposition. J. Phys. Chem. Lett. 2023, 14, 4696–4703. [Google Scholar] [CrossRef]
- Rasteiro, L.F.; Motin, M.A.; Vieira, L.H.; Assaf, E.M.; Zaera, F. Growth of ZrO2 films on mesoporous silica sieve via atomic layer deposition. Thin Solid Films 2023, 768, 139716. [Google Scholar] [CrossRef]
- Ke, W.; Liu, Y.; Wang, X.; Qin, X.; Chen, L.; Palomino, R.M.; Simonovis, J.P.; Lee, I.; Waluyo, I.; Rodriguez, J.A.; et al. Nucleation and Initial Stages of Growth during the Atomic Layer Deposition of Titanium Oxide on Mesoporous Silica. Nano Lett. 2020, 20, 6884–6890. [Google Scholar] [CrossRef]
- Zaera, F. Infrared Absorption Spectroscopy of Adsorbed CO: New Applications in Nanocatalysis for an Old Approach. ChemCatChem 2012, 4, 1525–1533. [Google Scholar] [CrossRef]
- Zaera, F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem. Soc. Rev. 2014, 43, 7624–7663. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.; Thompson, D. Gold-catalysed oxidation of carbon monoxide. Gold Bull. 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO Oxidation over Supported Gold Catalysts—“Inert” and “Active” Support Materials and Their Role for the Oxygen Supply during Reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Chen, M.; Goodman, D.W. Catalytically active gold on ordered titania supports. Chem. Soc. Rev. 2008, 37, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of Active Gold Nanoclusters on Iron Oxide Supports for CO Oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Xu, M.; Kunal, P.; Trewyn, B.G. Aerobic oxidative esterification of primary alcohols over Pd-Au bimetallic catalysts supported on mesoporous silica nanoparticles. Catal. Today 2018, 306, 81–88. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, C.; Yan, W.; Overbury, S.H.; Dai, S. Preparation of Highly Active Silica-Supported Au Catalysts for CO Oxidation by a Solution-Based Technique. J. Phys. Chem. B 2006, 110, 10842–10848. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, B.; Guerrero-Sánchez, J.; Lee, I.; Zhou, X.; Takeuchi, N.; Zaera, F. Controlling Selectivity in Unsaturated Aldehyde Hydrogenation Using Single-Site Alloy Catalysts. ACS Catal. 2019, 9, 9150–9157. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Ke, W.; Vazquez, Y.; Lee, I.; Zaera, F. CO Oxidation Catalyzed by Au Dispersed on SBA-15 Modified with TiO2 Films Grown via Atomic Layer Deposition (ALD). Catalysts 2023, 13, 1106. https://doi.org/10.3390/catal13071106
Qin X, Ke W, Vazquez Y, Lee I, Zaera F. CO Oxidation Catalyzed by Au Dispersed on SBA-15 Modified with TiO2 Films Grown via Atomic Layer Deposition (ALD). Catalysts. 2023; 13(7):1106. https://doi.org/10.3390/catal13071106
Chicago/Turabian StyleQin, Xiangdong, Wang Ke, Yovanny Vazquez, Ilkeun Lee, and Francisco Zaera. 2023. "CO Oxidation Catalyzed by Au Dispersed on SBA-15 Modified with TiO2 Films Grown via Atomic Layer Deposition (ALD)" Catalysts 13, no. 7: 1106. https://doi.org/10.3390/catal13071106