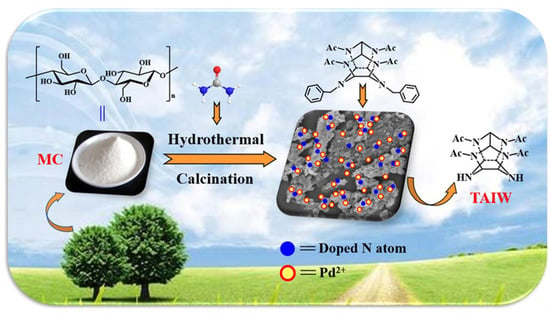
Structural Effects of Microcrystalline Cellulose-Derived Carbon Supports on Catalytic Performance of the Pd(OH)2/C Catalysts for the Hydrogenolytic Debenzylation of Hexanitrohexaazaisowurtzitane Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Carbon Supports
2.2. Catalytic Performance Characterization of the Catalysts
2.3. Characterization of the Catalysts
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Carbon Supports
3.3. Catalysts Preparation
3.4. Catalytic Activity Tests
3.5. Characterization and Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Theodoridis, G. Nitrogen protecting groups: Recent developments and new applications. Tetrahedron 2000, 56, 2339–2358. [Google Scholar] [CrossRef]
- Lange, S.; Formenti, D.; Lund, H.; Kreyenschulte, C.R.; Agostini, G. Additive-free nickel-catalysed debenzylation reactions via hydrogenative C-O and C-N bond cleavage. ACS Sustain. Chem. Eng. 2019, 7, 17107–17113. [Google Scholar] [CrossRef]
- Sandee, A.J.; Chintada, T.J.; Groen, C.; Donkervoort, J.G.; Terorde, R.J.A.M. Optimized palladium on activated carbon formulation for N-hydrogenolysis reactions. Chim. Oggi-Chem. Today 2013, 31, 20–23. [Google Scholar]
- Mao, J.F.; Gregory, D.H. Recent advances in the use of sodium borohydride as a solid state hydrogen store. Energies 2015, 8, 430–453. [Google Scholar] [CrossRef]
- Ram, S.; Spicer, L.D. Debenzylation of N-benzylamino derivatives by catalytic transfer hydrtyation with ammonium formate. Synth. Commun. 1987, 17, 415–418. [Google Scholar] [CrossRef]
- Watanabe, T.; Kobayashi, A.; Nishiura, M.; Takahashi, H.; Usui, T.; Kamiyama, I. Synthetic studies on indoles and related-compounds. 26. The debenzylation of protected indole nitrogen with aluminum-chloride. Chem. Pharm. Bull. 1991, 39, 1152–1156. [Google Scholar] [CrossRef] [Green Version]
- Rombouts, F.; Franken, D.; Martínez-Lamenca, C. Microwave-assisted N-debenzylation of amides with triflic acid. Tetrahedron Lett. 2010, 51, 4815–4818. [Google Scholar] [CrossRef]
- Kovacs, E.; Thurner, A.; Farkas, F. Hydrogenolysis of N-protected aminooxetanes over palladium: An efficient method for a one-step ring opening and debenzylation reaction. J. Mol. Catal. A Chem. 2011, 339, 32–36. [Google Scholar] [CrossRef]
- Nielsen, A.T.; Nissan, R.A.; Vanderah, D.J. Polyazapolycyclics by condensation of aldehydes with amines. 2. Formation of 2,4,6,8,10,12-hexabenzyl-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.05.9.03,11]dodecanes from glyoxal and benzylamines. J. Org. Chem. 1990, 55, 1459–1466. [Google Scholar] [CrossRef]
- Bellamy, A.J. Reductive debenzylation of hexabenzylhexaazaisowurtzitane. Tetrahedron 1995, 51, 4711–4722. [Google Scholar] [CrossRef]
- Nielsen, A.T.; Chafin, A.P.; Christian, S.L. Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 1998, 54, 11793–11812. [Google Scholar] [CrossRef]
- Wardle, R.B. Improved Hydrogenolysis of 2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-Hexaazatetracyclo [5.5.0.05,9.03,11] Dodecane. U.S. Patent 5,739,325, 14 April 1998. [Google Scholar]
- Yu, Y.Z.; Guan, X.P. Studies on the synthesis of hexanitrohexaazaisowurtzitane. Chin. J. Energetic Mater. 1999, 7, 1–4. [Google Scholar]
- Zheng, F.P.; Ou, Y.X.; Chen, J.T. The preparation and characterization of nanosized Pd(OH)2 and its application in the catalytic hydrogenolysis of HBIW. Chem. J. Chin. Univ. 1999, 20, 843–845. [Google Scholar]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Study of palladium catalyst deactivation in synthesis of 4,10-diformyl-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane. React. Kinet. Catal. Lett. 2007, 92, 293–302. [Google Scholar] [CrossRef]
- Zhang, M.X.; Liu, S.; Li, L. Effect of carbon supports on Pd catalyst for hydrogenation debenzylation of hexabenzylhexaaza- isowurtzitane (HBIW). J. Energ. Mater. 2017, 35, 251–264. [Google Scholar] [CrossRef]
- Koskin, A.P.; Simakova, I.L.; Parmon, V.N. Reductive debenzylation of hexabenzylhexaazaisowurtzitane—The key step of the synthesis of polycyclic nitramine hexanitrohexaazaisowurtzitane. Russ. Chem. Bull. Int. Ed. 2007, 56, 2370–2375. [Google Scholar] [CrossRef]
- Qiu, W.G.; Liu, H.B.; Dong, K. Preparation of Pd(OH)2/C catalyst for hydrogenolytic debenzylation of hexabenzylhexaazaisowurtzitane. Chin. J. Energ. Mater. 2014, 22, 441–446. [Google Scholar]
- Maksimowski, P.; Golofit, T.; Tomaszewski, W. Palladium catalyst in the HBIW hydrodebenzylation reaction. Deactivation and spent catalyst regeneration procedure. Cent. Eur. J. Energetic Mater. 2016, 13, 333–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotouhi-Far, F.; Bashiri, H.; Hamadanian, M. Study of deactivation of Pd(OH)2/C catalyst in reductive debenzylation of hexabenzylhexaazaisowurtzitane. Propellants Explos. Pyrotech. 2017, 42, 213–219. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, W.G.; Sun, J.Y. Synthesis of flowerlike carbon nanosheets from hydrothermally carbonized glucose: An in situ selfgenerating template strategy. RSC Adv. 2019, 9, 37355–37364. [Google Scholar] [CrossRef] [Green Version]
- Nan, J.P.; Wang, Y.L.; Song, J.W.; Qiu, W.G. Effects of the preparation condition of carbon support on the hydrogenolytic debenzylation performance of the corresponding Pd(OH)2/C catalysts. Chin. J. Energetic Mater. 2022, 30, 1138–1146. [Google Scholar]
- Zhang, Q.F.; Wang, M.; Qian, J.C.; Lou, S.Y.; Jin, J.H.; Li, B.C.; Lu, C.S.; Feng, F.; Lv, J.H.; Wang, Q.T.; et al. Deactivation and regeneration of palladium catalysts for hydrogenation debenzylation of 2,4,6,8,10,12-hexabenzyl-2,4,6,8,10,12-hexaazaisowurtzitane (HBIW). Catalysts 2022, 12, 1547. [Google Scholar] [CrossRef]
- Liu, S.; Ji, F.; Li, X.Y. Stick-like mesoporous titania loaded Pd as highly active and cost effective catalysts for hydrodebenzylation of hexabenzylhexaazaisowurtzitane (HBIW). Mol. Catal. 2019, 477, 110556. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Wang, H.S. Ultrasmall Pd nanoparticles supported on TiO2 for catalytic debenzylation via hydrogenative C-N bond cleavage. ACS Appl. Nano Mater. 2021, 4, 159–166. [Google Scholar] [CrossRef]
- Dong, K.; Chen, Y.; Zhang, Y.Y. The highly effective hydrogenolysis-based debenzylation of tetraacetyldibenzylhexaazaiso-wurtzitane (TADBIW) using a Palladium/DOWEX catalyst having a synergistic effect. J. Energ. Mater. 2017, 35, 421–429. [Google Scholar]
- Liu, W.; Li, S.F.; She, C.C. Excellent stability of Pd/mpg-C3N4 in catalytic hydrodebenzylation of 2, 4, 6,8,10,12-hexabenzy1-2,4,6,8,10,12-hexaazaisowurtzitane (HBIW). Appl. Catal. A-Gen. 2021, 624, 118310. [Google Scholar] [CrossRef]
- Lou, D.Y.; Wang, H.S.; Liu, S. PdFe bimetallic catalysts for debenzylation of hexabenzylhexaazaisowurtzitane (HBIW) and tetraacetyldibenzylhexaazaisowurtzitane (TADBIW). Catal. Commun. 2018, 109, 28–32. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, S.; Men, Y. Synergistic catalysis of PdFe bimetallic nanoparticles supported on SiO2 for hydrogenative cleavage of C-N bonds. ACS Appl. Nano Mater. 2021, 4, 6020–6029. [Google Scholar] [CrossRef]
- Cabiac, A.; Delahay, G.; Durand, R. The influence of textural and structural properties of Pd/carbon on the hydrogenation of cis,trans,trans-1,5,9-cyclododecatriene. Appl. Catal. A-Gen. 2007, 318, 17–21. [Google Scholar] [CrossRef]
- Cabiac, A.; Cacciaguerra, T.; Trens, P. Influence of textural properties of activated carbons on Pd/carbon catalysts synthesis for cinnamaldehyde hydrogenation. Appl. Catal. A-Gen. 2008, 340, 229–235. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.Y.; Tang, J.T.; Duan, Y.C. Conductive cellulose nanocrystals with high cycling stability for supercapacitor applications. J. Mater. Chem. A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Yi, X.T.; Liu, C.; Liu, X.K. Magnetic partially carbonized cellulose nanocrystal-based magnetic solid phase extraction for the analysis of triazine and triazole pesticides in water. Microchim. Acta 2019, 186, 825. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.; Karabulut, E.; Marais, A. Nanocellulose aerogels functionalized by rapid layer-by-layer assembly for high charge storage and beyond. Angew. Chem.-Int. Ed. 2013, 52, 12038–12042. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Z.; Zhou, J.; Nagaraju, D.H. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: Catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Jiang, Y.T.; Yan, J.; Wu, X.L. Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J. Power Source 2016, 307, 190–198. [Google Scholar] [CrossRef]
- Lu, H.; Sun, X.M.; Gaddam, R.R. Electrocapacitive properties of nitrogen-containing porous carbon derived from cellulose. J. Power Source 2017, 360, 634–641. [Google Scholar] [CrossRef]
- Lu, H.; Zhuang, L.Z.; Gaddam, R.R. Microcrystalline cellulose-derived porous carbons with defective sites for electrochemical applications. J. Mater. Chem. A 2019, 7, 22579–22587. [Google Scholar] [CrossRef]
- Paksung, N.; Pfersich, J.; Arauzo, P.J. Structural effects of cellulose on hydrolysis and carbonization behavior during hydrothermal treatment. ACS Omega 2020, 5, 12210–12223. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.L.; Qiu, J.H.; Jia, M.M. Platinum supported cellulose-based carbon with oxygen-containing functional groups for benzyl alcohol oxidation. J. Phys. Chem. Solids 2019, 135, 109095. [Google Scholar] [CrossRef]
- Xia, J.Q.; Cheng, M.; Hu, J. Zn5(OH)6(CO3)2-assisted controlled fabrication of microcrystalline cellulose-derived hierarchical porous carbon for high-performance lithium storage. J. Alloys Compd. 2022, 911, 165131. [Google Scholar] [CrossRef]
- Wu, X.S.; Dong, X.L.; Wang, B.Y. Revealing the sodium storage behavior of biomass-derived hard carbon by using pure lignin and cellulose as model precursors. Renew. Energy 2022, 189, 630–638. [Google Scholar] [CrossRef]
- Garba, Z.N.; Lawan, I.; Zhou, W.M. Microcrystalline cellulose (MCC) based materials as emerging adsorbents for the removal of dyes and heavy metals—A review. Sci. Total Environ. 2020, 717, 135070. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aust. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Kang, S.M.; Li, X.L.; Fan, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Z.C.; Yu, Q. Preparation of hierarchically porous carbon/magnetic particle composites with broad microwave absorption bandwidth. Chem. Eng. J. 2019, 359, 69–78. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, B.T.; Zhu, L.; Shao, Z.P. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Abakumov, A.A.; Bychko, I.B.; Selyshchev, O.V. Catalytic properties of reduced graphene oxide in acetylene hydrogenation. Carbon 2020, 157, 277–285. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H. Raman micro spectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Madalena, M.; Freitas, A. The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Zhou, J.H.; Sui, Z.J.; Zhu, J. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Cabiac, A.; Delahay, G.; Durand, R. Controlled preparation of Pd/AC catalysts for hydrogenation reactions. Carbon 2007, 45, 3–10. [Google Scholar] [CrossRef]
- An, N.H.; Dai, Y.S.; Tang, C. Design and preparation of a simple and effective palladium catalyst and the hydrogenation performance toward dibenzylbiotinmethylester. J. Colloid Interface Sci. 2016, 470, 56–61. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, L.M.; Ren, J.J. Facile synthesis of surface functionalized Pd2+@P-CDP/COFs for highly sensitive detection of norfloxacin drug based on the host-guest interaction. J. Pharm. Biomed. Anal. 2022, 219, 114956. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Schuster, M.E.; Xie, Z.L. Nature of the N-Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Koh, K.; Seo, J.E.; Lee, J.H. Ultrasmall palladium nanoparticles supported on amine-functionalized SBA-15 efficiently catalyze hydrogen evolution from formic acid. J. Mater. Chem. A 2014, 2, 20444–20449. [Google Scholar] [CrossRef]
- Abate, S.; Arrigo, R.; Schuster, M.E. Pd nanoparticles supported on N-doped nanocarbon for the direct synthesis of H2O2 from H2 and O2. Catal. Today 2010, 157, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, D.H. Hydrogen production from formic acid dehydrogenation over a Pd supported on N-doped mesoporous carbon catalyst: A role of nitrogen dopant. Appl. Catal. A-Gen. 2020, 608, 117887. [Google Scholar] [CrossRef]










| Sample | SBET (m2·g−1) | Smic (m2·g−1) | Vtot (m2·g−1) | Average Pore Width (nm) |
|---|---|---|---|---|
| HTC | 449.0 | 334.0 | 0.735 | 6.6 |
| HTC-N3:1 | 9.3 | 0.0 | 0.016 | 7.0 |
| HTC-N2:1 | 9.2 | 0.6 | 0.018 | 7.7 |
| HTC-N1:1 | 35.0 | 0.0 | 0.083 | 9.5 |
| HTC-N1:2 | 9.9 | 0.0 | 0.018 | 7.3 |
| Samples | Elemental Analysis (wt%) | |||
|---|---|---|---|---|
| N | C | H | O | |
| HTC | 0.3 | 91.3 | 1.8 | 6.6 |
| HTC-N3:1 | 7.6 | 82.6 | 1.4 | 8.4 |
| HTC-N2:1 | 8.2 | 81.9 | 1.2 | 8.7 |
| HTC-N1:1 | 8.8 | 80.9 | 1.4 | 8.9 |
| HTC-N1:2 | 9.6 | 77.0 | 1.3 | 12.1 |
| Number | Catalysts | Pd/Substrate (‰) | Results (%) | ||
|---|---|---|---|---|---|
| Pd/HBIW | Pd/TADB | TADB Yields | TADB Conversions | ||
| 1 | Pd/HTC | 1.4 | 2.0 | 90 | 94 |
| 2 | Pd/HTC-N3:1 | 1.4 | 2.0 | 89 | 64 |
| 3 | Pd/HTC-N2:1 | 1.4 | 2.0 | 89 | 75 |
| 4 | Pd/HTC-N1:1 | 1.4 | 2.0/2.35 | 90 | 97/100 |
| 5 | Pd/HTC-N1:2 | 1.4 | 2.0 | 89 | 88 |
| No. | Catalysts | Pd/Substrate (‰) | Results (%) | Ref. | ||
|---|---|---|---|---|---|---|
| Pd/HBIW | Pd/TADB | TADB Yields | TADB Conversions/TAIW Yields | |||
| 1 | Pd/ST-2.5 | 7.0 | - | 82 | - | [24] |
| 2 | Pd/TiO2-2 | 1.0 | 4.0 | 89 | 100/88 | [25] |
| 3 | Pd/mpg-C3N4 | 10.0 | - | 81.7 | - | [27] |
| 4 | Pd5Fe1/Si-CODP | 4.0 | 10.0 | 79 | 100/84 | [29] |
| 5 | Pd/HTC | 1.4 | 2.0 | 90 | 94/81 | this work |
| 6 | Pd/HTC-N1:1 | 1.4 | 2.0//2.35 | 90 | 97/82//100/87 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, Y.; Ding, X.; Song, J.; Wei, G.; Dai, H.; Wang, H.; Liu, Y.; Bai, G.; Qiu, W. Structural Effects of Microcrystalline Cellulose-Derived Carbon Supports on Catalytic Performance of the Pd(OH)2/C Catalysts for the Hydrogenolytic Debenzylation of Hexanitrohexaazaisowurtzitane Derivatives. Catalysts 2023, 13, 637. https://doi.org/10.3390/catal13030637
Wang Y, Chen Y, Ding X, Song J, Wei G, Dai H, Wang H, Liu Y, Bai G, Qiu W. Structural Effects of Microcrystalline Cellulose-Derived Carbon Supports on Catalytic Performance of the Pd(OH)2/C Catalysts for the Hydrogenolytic Debenzylation of Hexanitrohexaazaisowurtzitane Derivatives. Catalysts. 2023; 13(3):637. https://doi.org/10.3390/catal13030637
Chicago/Turabian StyleWang, Yuling, Yun Chen, Xinlei Ding, Jianwei Song, Gaixia Wei, Hengwei Dai, Hanyang Wang, Yadong Liu, Guangmei Bai, and Wenge Qiu. 2023. "Structural Effects of Microcrystalline Cellulose-Derived Carbon Supports on Catalytic Performance of the Pd(OH)2/C Catalysts for the Hydrogenolytic Debenzylation of Hexanitrohexaazaisowurtzitane Derivatives" Catalysts 13, no. 3: 637. https://doi.org/10.3390/catal13030637