Green Oxidative Catalytic Processes for the Preparation of APIs and Precursors
Abstract
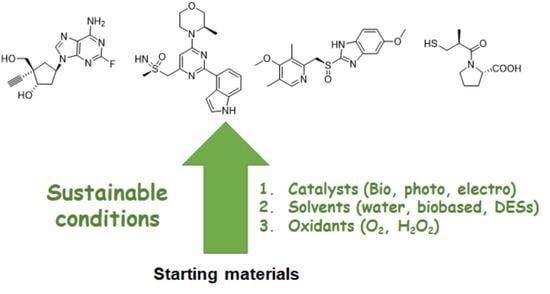
:1. Introduction
2. Biocatalytic Approaches for the Oxidative Preparation of APIs and Precursors
2.1. Oxidations Catalyzed by Monooxygenases
2.2. Other Biocatalyzed Oxidations
3. Other Sustainable Catalytic Asymmetric Oxidations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Food & Drug Administration Glossary of Terms. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms (accessed on 22 January 2023).
- Park, J.; Kelly, M.A.; Kang, J.X.; Seemakurti, S.S.; Ramirez, J.L.; Hatzell, M.C.; Sievers, C.; Bommarius, A.S. Production of active pharmaceutical ingredients (APIs) from lignin-derived phenol and catechol. Green Chem. 2021, 23, 7488–7498. [Google Scholar] [CrossRef]
- Horvath, I.T.; Anastas, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Fundamentals in Green Chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, P.T.; Williamson, T.C. Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- United Nation Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 22 January 2023).
- Campos, K.R.; Coleman, P.J.; Alvárez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.K.; Handa, S. A glimpse into Green Chemistry practices in the pharmaceutical industry. ChemSusChem 2020, 13, 2859–2875. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Application of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; Lankin, J.B.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Pillari, V.; Pace, V.; Alcántara, A.R.; de Gonzalo, G. Application of biobased solvents in asymmetric catalysis. Molecules 2022, 27, 6701. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Ielo, L.; Pillari, V.; Fernández, M.; Alcántara, A.R.; Pace, V. Biomass-Derived Solvents in Sustainable Organic Synthesis: Tools and Strategies; Protti, S., Palmieri, A., Eds.; Royal Society of Chemistry: Croydon, UK, 2022; pp. 239–279. [Google Scholar]
- Stuart, N.J.; Sanders, A.S. Phenyl Propionic Acids. US Patent 3385886, 2 February 1961. [Google Scholar]
- Andraos, J. Designing a green organic chemistry lecture course. In Green Organic Chemistry in Lecture and Laboratory; Dicks, A.P., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 2; pp. 29–68. [Google Scholar]
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic conversions in water. Part 5: Carbonylation of 1-(4-isobutylphenyl)- ethanol to ibuprofen catalysed by water-soluble palladium-phosphine complexes in a two-phase system. J. Chem. Technol. Biotechnol. 1997, 70, 83–91. [Google Scholar] [CrossRef]
- Trost, B.M. Atom Economy: A challenge for organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Vidaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Soloshonok, V.A.; Klika, K.D.; Drabowicz, J.; Wzorek, A. Chiral sulfoxides: Advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. [Google Scholar] [CrossRef]
- Wojaczynska, E.; Wojaczynski, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 2020, 120, 4578–4611. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 108, 801–838. [Google Scholar] [CrossRef]
- Domínguez de María, P.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as useful tool in asymmetric synthesis: An assessment of recently granted patents (2014–2019). Catalysts 2019, 9, 802. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Domínguez de María, P. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalysis for synthesis of pharmaceuticals. Bioorg. Med. Chem. 2018, 26, 1252–1274. [Google Scholar] [CrossRef] [PubMed]
- Simíc, S.; Zukíc, E.; Schmermund, L.; Faber, K.; Winkler, C.K.; Kroutil, W. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods. Chem. Rev. 2022, 122, 1052–1126. [Google Scholar] [CrossRef]
- Rossini, G.; Robescu, M.S.; Licastro, E.; Tedesco, C.; Martello, I.; Maffei, L.; Vincenti, G.; Bavaro, T.; Collina, S. Biocatalysis: A smart and green tool for the preparation of chiral drugs. Chirality 2022, 34, 1403–1418. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P.; Sánchez-Montero, J.M. Biocatalysis for the asymmetric synthesis of Active Pharmaceutical Ingredients (APIs): This time is for real. Expert Opin. Drug Discover. 2022, 17, 1159–1171. [Google Scholar] [CrossRef]
- Zawodny, W.; Montgomery, S.L. Evolving new chemistry: Biocatalysis for the synthesis of amine-containing pharmaceuticals. Catalysts 2022, 12, 595. [Google Scholar] [CrossRef]
- Colonna, S.; Del Sordo, S.; Gaggero, N.; Carrea, G.; Pasta, P. Enzyme-mediated catalytic asymmetric oxidations. Heteroat. Chem. 2002, 13, 467–473. [Google Scholar] [CrossRef]
- Dong, J.J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Torres-Pazmiño, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects, and biotechnological applications. J. Biotechnol. 2010, 146, 9–24. [Google Scholar] [CrossRef]
- Leisch, H.; Morley, K.; Lau, P.C.K. Baeyer-Villiger monooxygenases: More than just green chemistry. Chem. Rev. 2011, 111, 4165–4222. [Google Scholar] [CrossRef] [Green Version]
- Fürst, M.J.L.J.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer–Villiger monooxygenases: Tunable oxidative biocatalysts. ACS Catal. 2019, 9, 11207–11241. [Google Scholar] [CrossRef] [Green Version]
- De Gonzalo, G.; Alcántara, A.R. Multienzymatic processes involving Baeyer–Villiger monooxygenases. Catalysts 2021, 11, 605. [Google Scholar] [CrossRef]
- Foote, K.M.; Nissink, J.W.M.; Turner, P. Morpholinio Pyrimidines and Their Use in Therapy. Patent WO2011154737A, 11 June 2011. [Google Scholar]
- Goundry, W.R.F.; Adams, B.; Benson, H.; Demeritt, J.; McKown, S.; Mullholland, K.; Robertson, A.; Siedlecki, P.; Tomlin, P.; Vare, K. Development and scale-up of a biocatalytic process to form a chiral sulfoxide. Org. Process Res. Develop. 2017, 21, 107–113. [Google Scholar] [CrossRef]
- Lindberg, P.; Brändström, A.; Wallmark, B.; Mattsson, H.; Rikner, L.; Hoffman, K.-J. Omeprazole: The first proton pump inhibitor. Med. Res. Rev. 1990, 10, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E. Esomeprazole magnesium (Nexium). Rev. Gastroenterol. Disord. 2001, 1, 32–41. [Google Scholar]
- Cotton, H.; Elebring, T.; Larsson, M.; Li, L.; Sörensen, H.; von Unge, S. Asymmetric synthesis of esomeprazole. Tetrahedron: Asymmetry 2000, 11, 3819–3825. [Google Scholar] [CrossRef]
- Bong, Y.K.; Song, S.; Nazor, J.; Vogel, M.; Widegren, M.; Smith, D.; Collier, S.J.; Wilson, R.; Palanivel, S.M.; Narayanaswamy, K.; et al. Baeyer-Villiger monooxygenase-mediated synthesis of esomeprazole as an alternative for Kagan sulfoxidation. J. Org. Chem. 2018, 83, 7453–7458. [Google Scholar] [CrossRef]
- Stewart, J.D. Cyclohexanone monooxygenase: A useful reagent for asymmetric Baeyer-Villiger reactions. Curr. Org. Chem. 1998, 2, 195–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.-Q.; Xu, N.; Zhao, Q.; Yu, H.-L.; Xu, J.-H. Engineering of cyclohexanone monooxygenase for the enantioselective synthesis of (S)-omeprazole. ACS Sustain. Chem. Eng. 2019, 7, 7218–7226. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, J.; Wu, Y.-Q.; Zhang, Y.; Xia, J.-Y.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L. Enzymatic preparation of the chiral (S)-sulfoxide drug esomeprazole at pilot-scale levels. Org. Process Res. Develop. 2020, 24, 1124–1130. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Xu, N.; Wu, Y.-Q.; Zheng, Y.C.; Zhao, Q.; Lin, G.-Q.; Yu, H.-L.; Xu, J.-H. Discovery of two native Baeyer-Villiger monooxygenases for asymmetric synthesis of bulky chiral sulfoxides. Appl. Environ. Microbiol. 2018, 84, e00638-18. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Shou, C.; Geng, Q.; Zhao, C.; Xu, J.; Yu, H. A Baeyer-Villiger monooxygenase from Cupriavidus basilensis catalyzes asymmetric synthesis of (R)-lansoprazole and other pharmaco-sulfoxides. Appl. Microbiol. Biotechnol. 2021, 105, 3169–3180. [Google Scholar] [CrossRef]
- Urlacher, V.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic applications. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, Y.; Zhao, J.; Li, Q.; Yu, X.; Acevedo-Rocha, C.G.; Li, A. Bacterial cytochrome P450-catalyzed regio and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresur. Bioprocess. 2020, 7, 2. [Google Scholar] [CrossRef]
- Nahri, L.O.; Fulco, A.J. Characterization of a catalytically self-sufficient 119,000 dalton cytochrome P450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1986, 26, 7160–7169. [Google Scholar] [CrossRef]
- Acevedo-Rocha, C.G.; Gamble, C.; Lonsdale, R.; Li, A.; Nett, N.; Hoebenreich, S.; Deege, A. P450-catalyzed regio- and diastereoselective steroid hydroxylation: Efficient directed evolution enabled by mutability landscaping. ACS Catal. 2018, 8, 3395–3410. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, D.; Janocha, S.; Kiss, F.M.; Bernhardt, R. CYP106A2—A versatile biocatalyst with high potential for biotechnological production of selectively hydroxylated steroid and terpenoid compounds. BBA-Proteins Proteom. 2018, 1866, 11–22. [Google Scholar] [CrossRef]
- Nikolaus, J.; Nguyen, K.T.; Virus, C.; Riehm, J.L.; Hutter, M.; Bernhardt, R. Engineering of CYP106A2 for steroid 9α- and 6β-hydroxylation. Steroids 2017, 120, 41–48. [Google Scholar] [CrossRef]
- Litzenburger, M.; Kern, F.; Khatri, Y.; Bernhardt, R. Conversions of tricyclic antidepressants and antipsychotics with selected P450s from Sorangium cellulosum So ce56. Drug Metab. Dispos. 2015, 43, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Litzenburger, M.; Bernhardt, R. CYP260B1 acts as 9α-hydroxylase for 11-deoxycorticosterone. Steroids 2017, 127, 40–45. [Google Scholar] [CrossRef]
- Ohrui, H.; Kohgo, S.; Hayakawa, H.; Kodama, E.; Matsuoka, M.; Nakata, T.; Mitsuya, H. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: A nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucl. Nucl. Nucleic Acids 2007, 26, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, W.P.; de Gonzalo, G.; Mattevi, A.; Fraaije, M.W. Flavoprotein oxidases: Classification and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5177–5188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickl, M.; Fuchs, M.; Glueck, S.M.; Faber, K. The substrate tolerance of alcohol oxidases. Appl. Microbiol. Biotechnol. 2015, 16, 6617–6642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahart, A.J.C.; Staniland, J.; Miller, G.J.; Cosgrove, S.C. Oxidase enzymes as sustainable oxidation catalysts. R. Soc. Open Sci. 2022, 9, 211572. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Improving chemical synthesis using flow reactors. Expert Opin. Drug Discover. 2007, 2, 1487–1503. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.-E.; Kara, S. The rise of continuous flow biocatalysis—Fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Ötvos, S.B.; Kappe, O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advance interemediates. Green Chem. 2021, 23, 6117–6138. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A.; Cushman, D.W. Azetidine-2-carboxylic Acid Derivatives. US Patent 4046889, 6 September 1977. [Google Scholar]
- De Vitis, V.; Dall’Oglio, F.; Pinto, A.; De Micheli, C.; Molinari, F.; Conti, P.; Romano, D.; Tamborini, L. Chemoenzymatic synthesis in flow reactors: A rapid and convenient preparation of captopril. ChemistryOpen 2017, 6, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gandolfi, R.; Nitti, P.; Rollini, M.; Molinari, F. Acetic acid bacetria as enantioselective biocatalysts. J. Mol. Catal. B: Enzym. 2002, 17, 235–240. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D. Biotechnological and molecular approaches for vanillin production: A review. Appl. Biochem. Biotechnol. 2013, 169, 1353–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Guajardo, N.; Kara, S. Enzyme catalysis: In DES. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 257–272. [Google Scholar]
- Hasani, F.Z.I.M.; Amzazi, S.; Lavandera, I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules 2019, 24, 2190. [Google Scholar] [CrossRef] [Green Version]
- Czeisler, C.A.; Walsh, J.K.; Roth, T.; Hughes, R.J.; Wright, K.P.; Kingsbury, L.; Arora, S.; Schwartz, J.R.L.; Niebler, G.E.; Dinges, D.F. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N. Engl. J. Med. 2005, 353, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Pliszka, A.G. Modafinil: A review and its potential use in the treatment of long COVID fatigue and neurocognitive deficits. Am. J. Psychiatry Res. J. 2022, 17, 5–7. [Google Scholar] [CrossRef]
- Gao, F.; Wang, L.; Liu, Y.; Wang, S.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Enzymatic synthesis of (R)-modafinil by chloroperoxidase-catalyzed enantioselective sulfoxidation of 2-(diphenylmethylthio) acetamide. Biochem. Eng. J. 2015, 93, 243–249. [Google Scholar] [CrossRef]
- Torres-Duarte, C.; Vazquez-Duhalt, R. Applications and prospective of peroxidase biocatalysis in the environmental field. In Biocatalysis Based on Heme Peroxidases; Torres, E., Ayala, M., Eds.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Itoh, T. Ionic Liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- McAllister, G.D.; Parsons, A.F. Going green in process chemistry: Optimizing an asymmetric oxidation reaction to synthesize the antiulcer drug esomeprazole. J. Chem. Educ. 2019, 96, 2617–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A biomassderived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Petsi, M.; Zografos, A. 2,5-Diketopiperazine catalysts as activators of dioxygen in oxidative processes. ACS Catal. 2020, 10, 7093–7099. [Google Scholar] [CrossRef]
- Cicco, L.; Roggio, M.; López-Aguilar, M.; Ramos-Martín, M.; Perna, F.M.; García-Alvárez, J.; Vitale, P.; Capriati, V. Selective aerobic oxidation of alcohols in low melting mixtures and water and use for telescoped one-pot hybrid reaction. ChemistryOpen 2022, 11, e202200160. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Fraaije, M.W. Recent developments in flavin-based catalysis. ChemCatChem 2013, 5, 403–415. [Google Scholar] [CrossRef]
- Cibulka, R. Artificial Flavin systems for chemoselective and stereoselective oxidation. Eur. J. Org. Chem. 2015, 2015, 915–932. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kimura, M.; Kumoi, T.; Iida, H. Coupled flavin-iodine redox organocatalysts: Aerobic oxidative transformation from N-tosylhydrazones to 1,2,3-thiadiazoles. ACS Catal. 2017, 7, 4986–4989. [Google Scholar] [CrossRef]
- Iida, H.; Demizu, R.; Ohkado, R. Tandem flavin-iodine-catalyzed aerobic oxidative sulfenylation of imidazo[1,2-a]pyridines with thiols. J. Org. Chem. 2018, 83, 12291–12296. [Google Scholar] [CrossRef] [PubMed]
- Boerner, R.J.; Moller, H.J. Saripidem—A new treatment for panic disorders. Psychopharmakotherapie 1997, 4, 145–148. [Google Scholar]
- Hamdouchi, C.; de Blas, J.; del Prado, M.; Gruber, J.; Heinz, B.A.; Vance, L. 2-Amino-3-substituted-6-(E)-1-phenyl-2-(N-methylcarbamoyl)vinyl imidazo-[1,2-a]pyridines as a novel class of inhibitors of human rhinovirus: Stereospecific synthesis and antiviral activity. J. Med. Chem. 1999, 42, 50–59. [Google Scholar] [CrossRef]
- Dai, W.; Li, J.; Chen, B.; Li, G.; Lv, Y.; Wang, L.; Gao, S. Asymmetric oxidation catalysis by a phorphyrin-inspired manganese complex: High enantioselective sulfoxidation with a wide substrate scope. Org. Lett. 2013, 15, 5658–5661. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rakesh, K.P.; Ravindar, L.; Qin, H.-L. Visible-light initiated aerobic oxidations: A critical review. Green Chem. 2018, 20, 4790–4833. [Google Scholar] [CrossRef]
- Politano, F.; Oksdath-Mansilla, G. Light on the horizon: Current research and future perspectives in flow photochemistry. Org. Process Res. Develop. 2018, 22, 1045–1062. [Google Scholar] [CrossRef] [Green Version]
- Sambiagio, C.; Noël, T. Flow Photochemistry: Shine some light on those tubes! Trends Chem. 2020, 2, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Noël, T.; Zysman-Colman, E. The promise and pitfalls of photocatalysis for organic synthesis. Chem Catal. 2022, 2, 468–476. [Google Scholar] [CrossRef]
- Skolia, E.; Gkizis, P.L.; Nikitas, N.F.; Kokotos, C.G. Photochemical aerobic oxidation of sulfides to sulfoxides: The crucial role of wavelength irradiation. Green Chem. 2022, 24, 4108–4118. [Google Scholar] [CrossRef]
- Skolia, E.; Gkizis, P.L.; Kokotos, C.G. A sustainable photochemical aerobic sulfide oxidation: Access to sulforaphene and modafinil. Org. Biomol. Chem. 2022, 20, 5836–5844. [Google Scholar] [CrossRef]
- Juge, N.; Mitchen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Rafiee, M.; Konz, Z.M.; Graaf, M.D.; Koolman, H.F.; Stahl, S.S. Electrochemical oxidation of alcohols and aldehydes to carboxylic acids catalyzed by 4-acetamido-TEMPO: An alternative to “Anelli” and “Pinnick” oxidations. ACS Catal. 2018, 8, 6738–6744. [Google Scholar] [CrossRef]
- Cai, H.; Mangner, T.J.; Muzik, O.; Wang, M.-W.; Chugani, D.C.; Chugani, H.T. Radiosynthesis of 11C-levetiracetam: A potential marker for PET imaging of SV2A expression. ACS Med. Chem. Lett. 2014, 5, 1152–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Hoque, M.A.; Graaf, M.D.; Harper, K.C.; Wang, F.; Genders, J.D.; Stahl, S.S. Scalable Flow Electrochemical Alcohol Oxidation: Maintaining High Stereochemical Fidelity in the Synthesis of Levetiracetam. Org. Process Res. Develop. 2021, 25, 2601–2607. [Google Scholar] [CrossRef] [PubMed]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fernández, P.D.; Coto-Cid, J.M.; de Gonzalo, G. Green Oxidative Catalytic Processes for the Preparation of APIs and Precursors. Catalysts 2023, 13, 638. https://doi.org/10.3390/catal13030638
García-Fernández PD, Coto-Cid JM, de Gonzalo G. Green Oxidative Catalytic Processes for the Preparation of APIs and Precursors. Catalysts. 2023; 13(3):638. https://doi.org/10.3390/catal13030638
Chicago/Turabian StyleGarcía-Fernández, Pedro D., Juan M. Coto-Cid, and Gonzalo de Gonzalo. 2023. "Green Oxidative Catalytic Processes for the Preparation of APIs and Precursors" Catalysts 13, no. 3: 638. https://doi.org/10.3390/catal13030638