Pluronic-123 Assisted Synthesis of Cobalt Vanadate Microparticles (µ-CoV MPs) for Durable Electrochemical Oxygen Evolution Reaction in Seawater and Connate Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of µ-CoV MPs
2.1.1. Role of Ultrasonication in µ-CoV MPs Synthesis
2.1.2. Role of Pluronic (P-123) Surfactant in µ-CoV MPs Synthesis
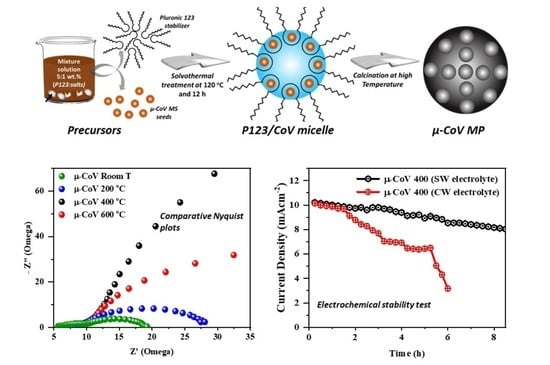
2.1.3. Role of Calcination Temperature in µ-CoV MPs Synthesis
2.2. Morphological and Structural Confirmation of µ-CoV MPs
2.3. XRD Patterns of µ-CoV MPs
2.4. Electrochemical Oxygen Evolution (OER) Performance
3. Experimental Section
3.1. Materials
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhao, C.; Muhmood, T.; Yang, X. Regulating the Assembly of Precursors of Carbon Nitrides to Improve Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1634. [Google Scholar] [CrossRef]
- Butt, F.K.; Tahir, M.; Cao, C.; Idrees, F.; Ahmed, R.; Khan, W.S.; Ali, Z.; Mahmood, N.; Tanveer, M.; Mahmood, A.; et al. Synthesis of Novel ZnV2O4 Hierarchical Nanospheres and Their Applications as Electrochemical Supercapacitor and Hydrogen Storage Material. ACS Appl. Mater. Interfaces 2014, 6, 13635–13641. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Mahmood, A.; Chen, S.G.; Sun, W.; Muhmood, T.; Yang, X.; Chen, Z. Solar-Driven Photocatalytic Reforming of Lignocellulose into H2 and Value-Added Biochemicals. ACS Catal. 2022, 12, 11206–11215. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.-K. Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Khan, I.; Qurashi, A. Shape Controlled Synthesis of Copper Vanadate Platelet Nanostructures, Their Optical Band Edges, and Solar-Driven Water Splitting Properties. Sci. Rep. 2017, 7, 14370. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Jalilov, A.; Fujii, K.; Qurashi, A. Quasi-1D Aligned Nanostructures for Solar-Driven Water Splitting Applications: Challenges, Promises, and Perspectives. Sol. RRL 2021, 5, 2000741. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Exner, K.S.; Over, H. Beyond the Rate-Determining Step in the Oxygen Evolution Reaction over a Single-Crystalline IrO2(110) Model Electrode: Kinetic Scaling Relations. ACS Catal. 2019, 9, 6755–6765. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Keeley, G.P.; Mayrhofer, K.J.J. A Comparative Study on Gold and Platinum Dissolution in Acidic and Alkaline Media. J. Electrochem. Soc. 2014, 161, H822–H830. [Google Scholar] [CrossRef]
- Khan, I.; Lee, J.H.; Park, J.; Wooh, S. Nano/micro-structural engineering of Nafion membranes for advanced electrochemical applications. J. Saudi Chem. Soc. 2022, 26, 101511. [Google Scholar] [CrossRef]
- Lutterman, D.A.; Surendranath, Y.; Nocera, D.G. A Self-Healing Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2009, 131, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.K.; Winkler, S.; Koch, N.; Pinna, N. Electrochemical water oxidation of ultrathin cobalt oxide-based catalyst supported onto aligned ZnO nanorods. ACS Appl. Mater. Interfaces 2016, 8, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science (80-) 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [Green Version]
- Mansha, M.; Khan, I.; Ullah, N.; Qurashi, A.; Sohail, M. Visible-light driven photocatalytic oxygen evolution reaction from new poly(phenylene cyanovinylenes). Dye. Pigment. 2017, 143, 95–102. [Google Scholar] [CrossRef]
- Fan, R.Y.; Xie, J.Y.; Yu, N.; Chai, Y.M.; Dong, B. Interface design and composition regulation of cobalt-based electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 10547–10572. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, Z.; Li, M.; Guan, J. Atomic cobalt catalysts for the oxygen evolution reaction. Chem. Commun. 2020, 56, 794–797. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.S.; Chen, H.M.; Hu, X. Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef]
- Haase, F.T.; Bergmann, A.; Jones, T.E.; Timoshenko, J.; Herzog, A.; Jeon, H.S.; Rettenmaier, C.; Cuenya, B.R. Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nat. Energy 2022, 7, 765–773. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Auer, A.A. Oxygen Evolution Reaction Electrocatalysis on Cobalt(oxy)hydroxide: Role of Fe Impurities. J. Phys. Chem. C 2022, 126, 18623–18635. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, N.; Ge, R.; Liu, J.; Li, W.; Chen, Y.; Feng, L.; Che, R. Porous Mn-doped cobalt phosphide nanosheets as highly active electrocatalysts for oxygen evolution reaction. Chem. Eng. J. 2021, 425, 131642. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Liu, Z.; Zhu, J. Synergy of copper doping and oxygen vacancies in porous CoOOH nanoplates for efficient water oxidation. Chem. Eng. J. 2021, 405, 126198. [Google Scholar] [CrossRef]
- Niu, W.; Shi, J.; Ju, L.; Li, Z.; Orlovskaya, N.; Liu, Y.; Yang, Y. Understanding Synergism of Cobalt Metal and Copper Oxide toward Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 12030–12040. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, J.; Zhang, J.; Qiu, L.; Chen, Y.; Zhong, C.; Han, X.; Deng, Y.; Hu, W. Regulating metal active sites of atomically-thin nickel-doped spinel cobalt oxide toward enhanced oxygen electrocatalysis. Chem. Eng. J. 2022, 435, 134261. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Cao, Y.; Wang, H.; Zhang, Y.; Zhang, S.; Li, Y.; Gong, H.; Liu, S.; Yang, Z.; et al. Understanding the Effect of Nickel Doping in Cobalt Spinel Oxides on Regulating Spin State to Promote the Performance of the Oxygen Reduction Reaction and Zinc-Air Batteries. ACS Energy Lett. 2022, 8, 159–168. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Baig, N.; Hendi, A.H.; Ehsan, M.F.; Sarfraz, N. A Bifunctional 2D Interlayered β-Cu2V2O7/Zn2V2O6 (CZVO) Heterojunction for Solar-Driven Nonsacrificial Dye Degradation and Water Oxidation. Energy Technol. 2021, 9, 2100034. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, I.; Sufyan, A.; Anjum, D.; Qurashi, A. Activation of Ni2V2O7 to nonstoichiometric NiV3O8 for solar-driven photoelectrochemical water oxidation. J. Environ. Chem. Eng. 2021, 9, 105526. [Google Scholar] [CrossRef]
- Khan, I.; Khan, A.A.Z.; Sufyan, A.; Khan, M.Y.; Inayath Basha, S.; Khan, A.A.Z. Ultrasonically controlled growth of monodispersed octahedral BiVO4 microcrystals for improved photoelectrochemical water oxidation. Ultrason. Sonochem. 2020, 68, 105233. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, S.; Mansha, M.; Qurashi, A. Sonochemical assisted hydrothermal synthesis of pseudo-flower shaped Bismuth vanadate (BiVO4) and their solar-driven water splitting application. Ultrason. Sonochem. 2017, 36, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Okuzono, T.; Toyotama, A.; Yamanaka, J. Mechanism of diffusiophoresis with chemical reaction on a colloidal particle. Phys. Rev. E 2019, 99, 012608. [Google Scholar] [CrossRef]
- Keh, H.J. Diffusiophoresis. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2008; pp. 365–369. [Google Scholar]
- Velegol, D.; Garg, A.; Guha, R.; Kar, A.; Kumar, M. Origins of concentration gradients for diffusiophoresis. Soft Matter 2016, 12, 4686–4703. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Dresp, S.; Ngo Thanh, T.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Yu, L.; Xiao, J.; Huang, C.; Zhou, J.; Qiu, M.; Yu, Y.; Ren, Z.; Chu, C.-W.; Yu, J.C. High-performance seawater oxidation by a homogeneous multimetallic layered double hydroxide electrocatalyst. Proc. Natl. Acad. Sci. USA 2023, 119, e2202382119. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, J.; Li, W.; Xu, H.; Zhang, C.; Qiu, X. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 3629. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, L.; Wang, M.; Qi, Y.; Guo, D.; Li, H.; Chen, X.; Wang, S. Transition Metal-Based Electrocatalysts for Seawater Oxidation. Adv. Mater. Interfaces 2022, 9, 2201486. [Google Scholar] [CrossRef]
- Xing, M.; Kong, L.-B.; Liu, M.-C.; Liu, L.-Y.; Kang, L.; Luo, Y.-C. Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J. Mater. Chem. A 2014, 2, 18435–18443. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Chen, J.; Guo, Q.; Wang, T.; Pang, H. Cobalt vanadium oxide thin nanoplates: Primary electrochemical capacitor application. Sci. Rep. 2015, 4, 5687. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhu, W.; Hua, L.; Yang, H.; Qiao, Y.; Zhang, R.; Guo, L.; Zhao, X.; Hou, Z. Ionic liquid-Pluronic P123 mixed micelle stabilized water-soluble Ni nanoparticles for catalytic hydrogenation. J. Colloid Interface Sci. 2014, 415, 117–126. [Google Scholar] [CrossRef]
- Barick, K.C.; Ekta; Gawali, S.L.; Sarkar, A.; Kunwar, A.; Priyadarsini, K.I.; Hassan, P.A. Pluronic stabilized Fe3O4 magnetic nanoparticles for intracellular delivery of curcumin. RSC Adv. 2016, 6, 98674–98681. [Google Scholar] [CrossRef]
- Khan, I.; Ibrahim, A.A.M.; Sohail, M.; Qurashi, A. Sonochemical assisted synthesis of RGO/ZnO nanowire arrays for photoelectrochemical water splitting. Ultrason. Sonochem. 2017, 37, 669–675. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I. Preparation and properties of single-walled carbon nanotubes/poly(butylene terephthalate) nanocomposites. Iran. Polym. J. 2014, 23, 53–58. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Chong, T.H.; Khaleduzzaman, S.S.; Shahrul, I.M.; Saidur, R.; Long, B.D.; Amalina, M.A. Effect of Ultrasonication Duration on Colloidal Structure and Viscosity of Alumina–Water Nanofluid. Ind. Eng. Chem. Res. 2014, 53, 6677–6684. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandit, A.B. Wastewater treatment: A novel energy efficient hydrodynamic cavitational technique. Ultrason. Sonochem. 2002, 9, 123–131. [Google Scholar] [CrossRef]
- Sakai, T.; Kurosawa, H.; Okada, T.; Mishima, S. Vesicle formation in mixture of a PEO-PPO-PEO block copolymer (Pluronic P123) and a nonionic surfactant (Span 65) in water. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 389, 82–89. [Google Scholar] [CrossRef] [Green Version]
- García-Pérez, U.M.; Martínez-De La Cruz, A.; Sepúlveda-Guzmán, S.; Peral, J. Low-temperature synthesis of BiVO4 powders by Pluronic-assisted hydrothermal method: Effect of the surfactant and temperature on the morphology and structural control. Ceram. Int. 2014, 40, 4631–4638. [Google Scholar] [CrossRef]
- Park, G.C.; Seo, T.Y.; Park, C.H.; Lim, J.H.; Joo, J. Effects of Calcination Temperature on Morphology, Microstructure, and Photocatalytic Performance of TiO2 Mesocrystals. Ind. Eng. Chem. Res. 2017, 56, 8235–8240. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef]
- Campbell, Z.S.; Baro, S.; Gao, Y.; Li, F.; Abolhasani, M. Flow Synthesis of Single and Mixed Metal Oxides. Chem. Methods 2022, 2, e202200007. [Google Scholar] [CrossRef]
- Dowding, I.; Hassani, M.; Sun, Y.; Veysset, D.; Nelson, K.A.; Schuh, C.A. Particle size effects in metallic microparticle impact-bonding. Acta Mater. 2020, 194, 40–48. [Google Scholar] [CrossRef]
- Suharyadi, E.; Pratiwi, S.H.; Indrayana, I.P.T.; Kato, T.; Iwata, S.; Ohto, K. Effects of annealing temperature on microstructural, magnetic properties, and specific absorption rate of Zn-Ni ferrite nanoparticles. Mater. Res. Express 2021, 8, 036101. [Google Scholar] [CrossRef]
- Mondal, A.; Ganguli, S.; Inta, H.R.; Mahalingam, V. Influence of Vanadate Structure on Electrochemical Surface Reconstruction and OER Performance of CoV2O6 and Co3V2O8. ACS Appl. Energy Mater. 2021, 4, 5381–5387. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, Y.; Liu, R.; Ma, Y.; Cao, K.; Kaiser, U.; Varzi, A.; Song, Y.-F.; Passerini, S.; Streb, C. Modular development of metal oxide/carbon composites for electrochemical energy conversion and storage. J. Mater. Chem. A 2019, 7, 13096–13102. [Google Scholar] [CrossRef]
- Yang, J.; Wu, M.; Gong, F.; Feng, T.; Chen, C.; Liao, J. Facile and controllable synthesis of solid Co3V2O8 micro-pencils as a highly efficient anode for Li-ion batteries. RSC Adv. 2017, 7, 24418–24424. [Google Scholar] [CrossRef] [Green Version]
- Laverock, J.; Chen, B.; Preston, A.R.H.; Smith, K.E.; Wilson, N.R.; Balakrishnan, G.; Glans, P.-A.; Guo, J.-H. Electronic structure of the kagome staircase compounds Ni3V2O8 and Co3V2O8. Phys. Rev. B 2013, 87, 125133. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrei, E.E.; Faggiani, R.; Calvo, C. Refinement of the crystal structure of Co3V2O8 and Ni3V2O8. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 2304–2306. [Google Scholar] [CrossRef]
- Nagy, E.; Vitai, M. Analysis of Mass Transport through Anisotropic, Catalytic/Bio-Catalytic Membrane Reactors. Catalysts 2019, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Alfath, M.; Lee, C.W. Recent Advances in the Catalyst Design and Mass Transport Control for the Electrochemical Reduction of Carbon Dioxide to Formate. Catalysts 2020, 10, 859. [Google Scholar] [CrossRef]
- Yu, L.; Sun, S.; Li, H.; Xu, Z.J. Effects of catalyst mass loading on electrocatalytic activity: An example of oxygen evolution reaction. Fundam. Res. 2021, 1, 448–452. [Google Scholar] [CrossRef]
- Pikula, T.; Szumiata, T.; Siedliska, K.; Mitsiuk, V.I.; Panek, R.; Kowalczyk, M.; Jartych, E. The Influence of Annealing Temperature on the Structure and Magnetic Properties of Nanocrystalline BiFeO3 Prepared by Sol–Gel Method. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 470–483. [Google Scholar] [CrossRef]
- Hao, M.; Xiao, M.; Qian, L.; Miao, Y. Synthesis of cobalt vanadium nanomaterials for efficient electrocatalysis of oxygen evolution. Front. Chem. Sci. Eng. 2018, 12, 409–416. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Saeed, A.H.; Sharif, M.; Rehman, A. Direct deposition of amorphous cobalt-vanadium mixed oxide films for electrocatalytic water oxidation. ACS Omega 2019, 4, 12671–12679. [Google Scholar] [CrossRef]
- Keerthana, S.; Yuvakkumar, R.; Ravi, G.; Pannipara, M.; Al-Sehemi, A.G.; Velauthapillai, D. Cobalt Vanadium Oxide Nanoclusters for Oxygen Evolution Reaction. ECS J. Solid State Sci. Technol. 2021, 10, 071003. [Google Scholar] [CrossRef]
- Xiao, G.; Chen, W.; Cai, Y.; Zhang, S.; Wang, D.; Cai, D. Facile Synthesis of Sulfate-Intercalated CoFe LDH Nanosheets Derived from Two-Dimensional ZIF-9(III) for Promoted Oxygen Evolution Reaction. Catalysts 2022, 12, 688. [Google Scholar] [CrossRef]
- Bai, X.; Duan, Z.; Nan, B.; Wang, L.; Tang, T.; Guan, J. Unveiling the active sites of ultrathin Co-Fe layered double hydroxides for the oxygen evolution reaction. Chin. J. Catal. 2022, 43, 2240–2248. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Applications of single-atom catalysts. Nano Res. 2021, 15, 38–70. [Google Scholar] [CrossRef]
- Chen, D.; Pan, L.; Pei, P.; Song, X.; Ren, P.; Zhang, L. Cobalt-based oxygen electrocatalysts for zinc-air batteries: Recent progress, challenges, and perspectives. Nano Res. 2022, 15, 5038–5063. [Google Scholar] [CrossRef]
- Gyanprakash, D.M.; Sharma, G.P.; Gupta, P.K. Isovalent anion-induced electrochemical activity of doped Co3V2O8 for oxygen evolution reaction application. Dalt. Trans. 2022, 51, 15312–15321. [Google Scholar] [CrossRef]
- Cao, C. nan On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—II. Two state variables besides electrode potential. Electrochim. Acta 1990, 35, 837–844. [Google Scholar] [CrossRef]
- Schwab, M.J.; Han, J.; Pfefferle, L.D. Neutral anodic etching of GaN for vertical or crystallographic alignment. Appl. Phys. Lett. 2015, 106, 241603. [Google Scholar] [CrossRef]
- Hou, X.; Gao, L.; Cui, Z.; Yin, J. Corrosion and Protection of Metal in the Seawater Desalination. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022037. [Google Scholar] [CrossRef] [Green Version]
- Lukatskaya, M.R.; Halim, J.; Dyatkin, B.; Naguib, M.; Buranova, Y.S.; Barsoum, M.W.; Gogotsi, Y.; Lukatskaya, M.R.; Halim, J.; Dyatkin, B.; et al. Room-Temperature Carbide-Derived Carbon Synthesis by Electrochemical Etching of MAX Phases. Angew. Chemie 2014, 126, 4977–4980. [Google Scholar] [CrossRef]
- Zhou, L.; Bo, B.; Yan, X.; Wang, C.; Chi, Y.; Yang, X. Brief Review of Surface Passivation on III-V Semiconductor. Catalysts 2018, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Azeim, S.; Sakthivel, S.; Kandiel, T.A.; Kanj, M.Y. Specificity and Synergy at the Oil–Brine Interface: New Insights from Experiments and Molecular Dynamics Simulations. Energy Fuels 2021, 35, 14647–14657. [Google Scholar] [CrossRef]
- Zaeri, M.R.; Shahverdi, H.; Hashemi, R.; Mohammadi, M. Impact of water saturation and cation concentrations on wettability alteration and oil recovery of carbonate rocks using low-salinity water. J. Pet. Explor. Prod. Technol. 2019, 9, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Feiner, A.-S.; McEvoy, A.J. The Nernst Equation. J. Chem. Educ. 1994, 71, 493. [Google Scholar] [CrossRef]





| Type of Metal Vanadate | The Weight Ratio of Cobalt:Vanadium Precursors | Surfactant to Metal Precursor Weight Ratio | Reaction Temperature and Time | Physical Appearance |
|---|---|---|---|---|
| Co3(VO4)2 | 3:2 | 1:5 | 120 °C for 12 h | Brown @ <200 °C |
| (µ-CoV MPs) | Black @ >200 °C |
| Metal Vanadate (Code) | XRD Entry # (Phase Type) | Formula Unit | Ref. |
|---|---|---|---|
| Cobalt vanadate (µ-CoV MPs) | 00-016-0675 (orthorhombic) | Co3(VO4)2 | [40,55,56] |
| Sample | Specific Surface Area | Pore Size (BJH) | Pore Volume (BJH) |
|---|---|---|---|
| µ-CoV MPs | 131.22 m2 g−1 | 5.8 nm | 0.18 nm |
| Electrode Material [Ref.] | Morphology and Particles Size | Synthesis Method | Electrolyte | VOER@ 10 mA | Stability |
|---|---|---|---|---|---|
| Co3V2O8 MPs (This work) | globular shaped particles of 3–5 µm size | Pluronic-123 assisted solvothermal synthesis | Seawater | 1.557 | Sustained >80% OER stability after 8 h, while activity lost <1% after 1000 LSV cycles. |
| Co3V2O8 MPs (This work) | globular shaped particles of 3-–5 µm size | Pluronic-123 assisted solvothermal synthesis | Connate water | 1.557 | Not sustained OER activity after 2 h, while activity lost >10% after 1000 LSV cycles. |
| Co3V2O8 NPs [39] | Irregular nanoparticles of average size 20 nm | Stirring-assisted Wet chemistry synthesis | 1 M KOH | 1.595 | Stable for 3 h |
| Co3V2O8 nanosheets [63] | Ultrafine 2D-nanosheets at the nanoscale level | DMF, ethanol and glycol solvent-derived synthesis | 1 M KOH | 1.563 | Optimized catalyst lost 23% activity after 150 CV cycles |
| Co3V2O8 nanosheets [54] | Irregular spheres with diameters ranging from 40 to 250 nm | Sol-gel method followed by calcination | 1 M KOH | 1.74 | The stability results for Co3V2O8 are not provided. |
| Co–V mixed oxide catalysts (CoVOx) [64] | A network of interwoven nanofibers appeared at the nanoscale level | Aerosol assisted chemical vapor deposition synthesis | 0.5 M KOH | 1.54@ 20 mA | Stable for 6 h, while <1% activity loss after 500 cycles. |
| Co and V mixed at 1:1 [65] | Nanoclusters formed by large agglomeration | Chemical mixing and high-temperature annealing | 1 M KOH | 0.43 vs. Ag/AgCl | Stable for18 h |
| Component Salts | Connate Water (g/L) | Seawater (g/L) |
|---|---|---|
| NaHCO3 | 0.487 | 0.165 |
| Na2SO4 | 0.518 | 6.339 |
| NaCl | 150.446 | 41.17 |
| CaCl2·2H2O | 69.841 | 2.387 |
| MgCl2·6H2O | 20.396 | 17.416 |
| Total | 241.688 | 67.480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I. Pluronic-123 Assisted Synthesis of Cobalt Vanadate Microparticles (µ-CoV MPs) for Durable Electrochemical Oxygen Evolution Reaction in Seawater and Connate Water. Catalysts 2023, 13, 636. https://doi.org/10.3390/catal13030636
Khan I. Pluronic-123 Assisted Synthesis of Cobalt Vanadate Microparticles (µ-CoV MPs) for Durable Electrochemical Oxygen Evolution Reaction in Seawater and Connate Water. Catalysts. 2023; 13(3):636. https://doi.org/10.3390/catal13030636
Chicago/Turabian StyleKhan, Ibrahim. 2023. "Pluronic-123 Assisted Synthesis of Cobalt Vanadate Microparticles (µ-CoV MPs) for Durable Electrochemical Oxygen Evolution Reaction in Seawater and Connate Water" Catalysts 13, no. 3: 636. https://doi.org/10.3390/catal13030636