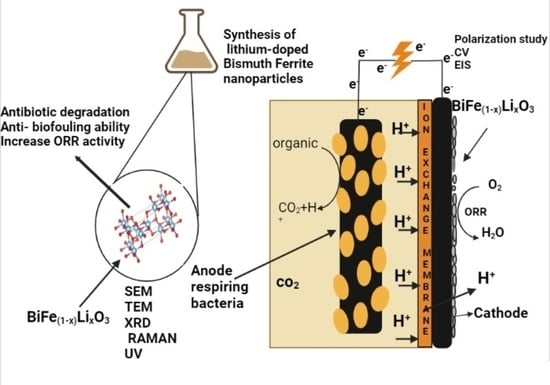
Investigating the Performance of Lithium-Doped Bismuth Ferrite [BiFe1−xLixO3]-Graphene Nanocomposites as Cathode Catalyst for the Improved Power Output in Microbial Fuel Cells
Abstract
:1. Introduction
2. Results
2.1. XRD Analysis
2.2. FESEM Analysis
2.3. EDXS Analysis
2.4. Raman Spectra Analysis
2.5. FTIR Analysis
2.6. Optical Properties
2.7. Photocatalytic Activity
2.8. Electrochemical ORR Activity of [BiFe1−xLixO3]-graphene Composite Electrodes
2.9. Power Generation from M.F.C. Having [BiFe1−xLixO3]–graphene Composite Loaded Cathode
2.10. EIS Analysis for Charge Transfer Resistance
2.11. Evaluation of Biofouling on the Different Concentrations of [BiFe1−xLixO3]-Loaded Graphene Composite Cathode Surface
3. Materials and Methods
3.1. Synthesis of [BiFe1−xLixO3]-Graphene Nanocomposite
3.2. Characterization of [BiFe1−xLixO3]-Graphene Nanocomposite
3.3. Electrochemical Study for [BiFe1−xLixO3]-Graphene Composite-Loaded Cathode
3.4. Construction of MFC
3.5. Inoculum and Anolyte Preparation
3.6. Performance Evaluation of MFCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A Survey of Photocatalytic Materials for Environmental Remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Li, F.; Sharma, Y.; Lei, Y.; Li, B.; Zhou, Q. Microbial Fuel Cells: The Effects of Configurations, Electrolyte Solutions, and Electrode Materials on Power Generation. Appl. Biochem. Biotechnol. 2010, 160, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Chapter 9—Noble Metals. In Membrane-Based Separations in Metallurgy; Jiang, L.Y., Li, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 249–272. ISBN 978-0-12-803410-1. [Google Scholar]
- Liu, Z.; Wu, H.; Ren, W.; Ye, Z.-G. Piezoelectric and Ferroelectric Materials: Fundamentals, Recent Progress, and Applications. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-409547-2. [Google Scholar]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional Manganese Ferrite/Polyaniline Hybrid as Electrode Material for Enhanced Energy Recovery in Microbial Fuel Cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical Conductivity of Conductive Carbon Blacks: Influence of Surface Chemistry and Topology. Appl. Surf. Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent Developments in Heterogeneous Photocatalytic Water Treatment Using Visible Light-Responsive Photocatalysts: A Review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. A Review on the Visible Light Active BiFeO3 Nanostructures as Suitable Photocatalyst in the Degradation of Different Textile Dyes. Environ. Nanotechnol. Monit. Manag. 2017, 7, 110–120. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic Activity and Doping Effects of BiFeO3 Nanoparticles in Model Organic Dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, F.; Gao, T.; Zhang, N.; Chen, Z.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Hydrothermal Synthesis of BiFeO3 Nanoparticles for Visible Light Photocatalytic Applications. J. Nanosci. Nanotechnol. 2015, 15, 9693–9698. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Hao, C.; Dong, X.; Guo, T.; Zhang, L.; Li, M.; Wang, H. Tunable multiferroic and bistable/complementary resistive switching properties of dilutely Li-doped BiFeO3 nanoparticles: An effect of aliovalent substitution. Nanoscale 2014, 6, 4735–4744. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Yokomizo, Y.; Krishnamurthy, S.; Kamat, P. Photoinduced Electron Charge and Discharge of Graphene–ZnO Nanoparticle Assembly. Catal. Today 2013, 199, 36–41. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Sono-Synthesis of Nanocrystalized BiFeO3/Reduced Graphene Oxide Composites for Visible Photocatalytic Degradation Improvement of Bisphenol A. Chem. Eng. J. 2016, 306, 204–213. [Google Scholar] [CrossRef]
- Seehra, M.S.; Geddam, U.K.; Schwegler-Berry, D.; Stefaniak, A.B. Detection and quantification of 2H and 3R phases in commercial graphene-based materials. Carbon 2015, 95, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A. Facile Synthesis of Mesoporous BiFeO3/Graphene Nanocomposites as Highly Photoactive under Visible Light. Opt. Mater. 2020, 104, 109842. [Google Scholar] [CrossRef]
- Hermet, P.; Goffinet, M.; Kreisel, J.; Ghosez. Raman and Infrared Spectra of Multiferroic Bismuth Ferrite from First Principles. Phys. Rev. B 2007, 75, 220102. [Google Scholar] [CrossRef]
- Bielecki, J.; Svedlindh, P.; Tibebu, D.T.; Cai, S.; Eriksson, S.G.; Börjesson, L.; Knee, C.S. Structural and magnetic properties of isovalently substituted multiferroic BiFeO3: Insights from Raman spectroscopy. Phys. Rev. B 2012, 86, 184422. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xu, X.; Koenigsmann, C.; Santulli, A.C.; Wong, S.S.; Musfeldt, J.L. Size-Dependent Infrared Phonon Modes and Ferroelectric Phase Transition in BiFeO3 Nanoparticles. Nano Lett. 2010, 10, 4526–4532. [Google Scholar] [CrossRef]
- Chauhan, S.; Arora, M.; Sati, P.C.; Chhoker, S.; Katyal, S.C.; Kumar, M. Structural, vibrational, optical, magnetic and dielectric properties of Bi1−xBaxFeO3 nanoparticles. Ceram. Int. 2013, 39, 6399–6405. [Google Scholar] [CrossRef]
- Seehra, M.S.; Narang, V.; Geddam, U.K.; Stefaniak, A.B. Correlation between X-ray Diffraction and Raman Spectra of 16 Commercial Graphene-Based Materials and Their Resulting Classification. Carbon 2017, 111, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Xian, T.; Yang, H.; Di, L.; Ma, J.; Zhang, H.; Dai, J. Photocatalytic reduction synthesis of SrTiO3-graphene nanocomposites and their enhanced photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Rashid, N.; Hu, N.; Rehman, M.S.U.; Han, J.-I. Electricity Generation and Microalgae Cultivation in Microbial Fuel Cell Using Microalgae-Enriched Anode and Bio-Cathode. Energy Convers. Manag. 2014, 79, 674–680. [Google Scholar] [CrossRef]
- Sharma, K.; Pandit, S.; Thapa, B.S.; Pant, M. Biodegradation of Congo Red Using Co-Culture Anode Inoculum in a Microbial Fuel Cell. Catalysts 2022, 12, 1219. [Google Scholar] [CrossRef]
- Rossi, R.; Cario, B.P.; Santoro, C.; Yang, W.; Saikaly, P.E.; Logan, B.E. Evaluation of Electrode and Solution Area-Based Resistances Enables Quantitative Comparisons of Factors Impacting Microbial Fuel Cell Performance. Environ. Sci. Technol. 2019, 53, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Application of Graphene in Electronic Devices|Encyclopedia MDPI. Available online: https://encyclopedia.pub/entry/29675 (accessed on 9 December 2022).
- Schulze, M.C.; Neale, N.R. Half-Cell Cumulative Efficiency Forecasts Full-Cell Capacity Retention in Lithium-Ion Batteries. ACS Energy Lett. 2021, 6, 1082–1086. [Google Scholar] [CrossRef]
- Open Circuit Potential (OCP). Pine Research Instrumentation Store; Pine Research Instrumentation: Durham, NC, USA, 2019. [Google Scholar]
- Kolajo, O.O.; Pandit, C.; Thapa, B.S.; Pandit, S.; Mathuriya, A.S.; Gupta, P.K.; Jadhav, D.A.; Lahiri, D.; Nag, M.; Upadhye, V.J. Impact of Cathode Biofouling in Microbial Fuel Cells and Mitigation Techniques. Biocatal. Agric. Biotechnol. 2022, 43, 102408. [Google Scholar] [CrossRef]
- Vempaty, A.; Kumar, A.; Pandit, S.; Gupta, M.; Mathuriya, A.S.; Lahiri, D.; Nag, M.; Kumar, Y.; Joshi, S.; Kumar, N. Evaluation of the Datura Peels Derived Biochar-Based Anode for Enhancing Power Output in Microbial Fuel Cell Application. Biocatal. Agric. Biotechnol. 2023, 47, 102560. [Google Scholar] [CrossRef]
- Pandit, S.; Khanna, S.; Mathuriya, A.S. Analysis of Pyridine-2-Carbaldehyde Thiosemicarbazone as an Anti-Biofouling Cathodic Agent in Microbial Fuel Cell. Appl. Microbiol. Biotechnol. 2023, 107, 459–472. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, A.; Pandit, S.; Vempaty, A.; Kumar, M.; Thapa, B.S.; Rai, N.; Peera, S.G. Investigating the Performance of a Zinc Oxide Impregnated Polyvinyl Alcohol-Based Low-Cost Cation Exchange Membrane in Microbial Fuel Cells. Membranes 2023, 13, 55. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Das, D.; Pradhan, D. Graphene Supported α-MnO2 Nanotubes as a Cathode Catalyst for Improved Power Generation and Wastewater Treatment in Single-Chambered Microbial Fuel Cells. RSC Adv. 2013, 3, 7902–7911. [Google Scholar] [CrossRef]
- Kumar, A.; Narayanan, S.S.; Thapa, B.S.; Pandit, S.; Pant, K.; Mukhopadhyay, A.K.; Peera, S.G. Application of Low-Cost Plant-Derived Carbon Dots as a Sustainable Anode Catalyst in Microbial Fuel Cells for Improved Wastewater Treatment and Power Output. Catalysts 2022, 12, 1580. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, V.; Pandit, S.; Thapa, B.S.; Pant, K.; Tusher, T.R. Isolation of Biosurfactant-Producing Bacteria and Their Co-Culture Application in Microbial Fuel Cell for Simultaneous Hydrocarbon Degradation and Power Generation. Sustainability 2022, 14, 15638. [Google Scholar] [CrossRef]
- Tripathi, B.; Pandit, S.; Sharma, A.; Chauhan, S.; Mathuriya, A.S.; Dikshit, P.K.; Gupta, P.K.; Singh, R.C.; Sahni, M.; Pant, K.; et al. Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts 2022, 12, 1040. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pema, T.; Kumar, A.; Tripathi, B.; Pandit, S.; Chauhan, S.; Singh, S.; Dikshit, P.K.; Mathuriya, A.S.; Gupta, P.K.; Lahiri, D.; et al. Investigating the Performance of Lithium-Doped Bismuth Ferrite [BiFe1−xLixO3]-Graphene Nanocomposites as Cathode Catalyst for the Improved Power Output in Microbial Fuel Cells. Catalysts 2023, 13, 618. https://doi.org/10.3390/catal13030618
Pema T, Kumar A, Tripathi B, Pandit S, Chauhan S, Singh S, Dikshit PK, Mathuriya AS, Gupta PK, Lahiri D, et al. Investigating the Performance of Lithium-Doped Bismuth Ferrite [BiFe1−xLixO3]-Graphene Nanocomposites as Cathode Catalyst for the Improved Power Output in Microbial Fuel Cells. Catalysts. 2023; 13(3):618. https://doi.org/10.3390/catal13030618
Chicago/Turabian StylePema, Tenzin, Ankit Kumar, Babita Tripathi, Soumya Pandit, Sunil Chauhan, Satyendra Singh, Pritam Kumar Dikshit, Abhilasha Singh Mathuriya, Piyush Kumar Gupta, Dibyajit Lahiri, and et al. 2023. "Investigating the Performance of Lithium-Doped Bismuth Ferrite [BiFe1−xLixO3]-Graphene Nanocomposites as Cathode Catalyst for the Improved Power Output in Microbial Fuel Cells" Catalysts 13, no. 3: 618. https://doi.org/10.3390/catal13030618