Scale-Up Lipase Production and Development of Methanol Tolerant Whole-Cell Biocatalyst from Magnusiomyces spicifer SPB2 in Stirred-Tank Bioreactor and Its Application for Biodiesel Production
Abstract
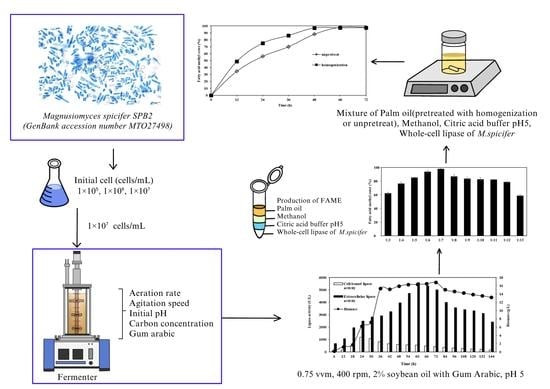
:1. Introduction
2. Results and Discussion
2.1. Effects of Inoculum Size on CBL Extracellular Lipase, and Biomass from M. spicifer SPB2 in Shake-Flask Cultivation
2.2. Stirred-Tank Bioreactor Operation
2.2.1. Effects of Aeration Rates on CBL, Extracellular Lipase, and Biomass Production from M. spicifer SPB2
2.2.2. Effects of Agitation Speeds on CBL, Extracellular Lipase, and Biomass Production from M. spicifer SPB2
2.2.3. Effects of Soybean Oil Concentrations on CBL, Extracellular Lipase, and Biomass Production from M. spicifer SPB2
2.2.4. Effects of Gum Arabic on CBL, Extracellular Lipase, and Biomass Production from M. spicifer SPB2
2.2.5. Effects of Initial pH for Cultivation on CBL and Extracellular Lipase Activity from M. spicifer SPB2
2.2.6. CBL, Extracellular Lipase, and Biomass Production from M. spicifer SPB2 under the Optimized Condition of Cultivation in a 5 L Stirred-Tank Bioreactor
2.3. FAME Production Using Whole-Cell Lipase from M. spicifer SPB2 Cultured in a Bioreactor
3. Materials and Methods
3.1. Microorganism, Cultivation and Inoculum Preparation
3.2. Effects of Inoculum Size on Lipase Production from M. spicifer SPB2
3.3. Stirred-Tank Bioreactor Operation
3.4. Transesterification Reaction for Biodiesel Production
3.4.1. Effects of Palm Oil and Methanol Molar Ratios on FAME Yield Produced through Transesterification Reaction Catalyzed by Whole-Cell Biocatalyst from M. spicifer SPB2
3.4.2. Effect of Homogenization Pretreatment on FAME Yield Produced through Transesterification Reaction Catalyzed by Whole-Cell Biocatalyst from M. spicifer SPB2
3.5. Determination of Lipase Activity
3.6. Determination of Biomass Production
3.7. Gas Chromatography Analysis for FAME
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaeger, K.E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, K.; Liu, H.; Jiang, Y.; Wang, R.; Wang, W.; Wang, T. A valuable product of microbial cell factories: Microbial lipase. Front. Microbiol. 2021, 12, 743377. [Google Scholar] [CrossRef] [PubMed]
- Colacicco, M.; Ciliberti, C.; Biundo, A.; Agrimi, G.; Pisano, I. Study of lipase production by Yarrowia lipolytica grown in high concentration of hydrophobic carbon sources. Chem. Eng. Trans. 2022, 93, 247–252. [Google Scholar]
- Elibol, M.; Ozer, D. Influence of oxygen transfer on lipase production by Rhizopus arrhizus. Process Biochem. 2000, 36, 325–329. [Google Scholar] [CrossRef]
- Aghabeigi, F.; Nikkhah, H.; Zilouei, H.; Bazarganipour, M. Immobilization of lipase on the graphene oxides magnetized with NiFe2O4 nanoparticles for biodiesel production from microalgae lipids. Process Biochem. 2023, 126, 171–185. [Google Scholar] [CrossRef]
- Al Mohaini, M.A.; Farid, A.; Muzammal, M.; Ghazanfar, S.; Dadrasnia, A.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N.; Ismail, S. Enhancing lipase production of Bacillus salmalaya strain 139SI using different carbon sources and surfactants. Appl. Microbiol. 2022, 2, 237–247. [Google Scholar] [CrossRef]
- Tembhurkar, V.R.; Kulkarni, M.B.; Peshwe, S.A. Optimization of lipase production by Pseudomonas spp. in submerged batch process in shake flask culture. Sci. Res. Rep. 2012, 2, 46–50. [Google Scholar]
- Haniya, M.; Naaz, A.; Sakhawat, A.; Amir, S.; Zahid, H.; Syed, S.A. Optimized production of lipase from Bacillus subtilis PCSIRNL-39. Afr. J. Biotechnol. 2017, 16, 1106–1115. [Google Scholar] [CrossRef] [Green Version]
- Ayinla, Z.A.; Ademakinwa, A.N.; Agboola, F.K. Studies on the optimization of lipase production by Rhizopus sp. ZAC3 isolated from the contaminated soil of a palm oil processing shed. J. Appl. Biol. Biotechnol. 2017, 5, 030–037. [Google Scholar]
- Colla, L.M.; Primaz, A.L.; Benedetti, S.; Loss, R.A.; de Lima, M.; Reinehr, C.O.; Bertolin, T.E.; Costa, J.A.V. Surface response methodology for the optimization of lipase production under submerged fermentation by filamentous fungi. Braz. J. Microbiol. 2016, 47, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, S.; Chhabra, M. Isolation, identification and characterization of Cystobasidium oligophagum JRC1: A cellulase and lipase producing oleaginous yeast. Bioresour. Technol. 2017, 223, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.; Ucar, M.H.; Unver, Y.; Kara, A.A.; Ozdemir, M.O.; Ortucu, S. Lipase production with free and immobilized cells of cold-adapted yeast Rhodotorula glutinis HL25. Biocatal. Agric. Biotechnol. 2016, 8, 97–103. [Google Scholar] [CrossRef]
- Joshi, R.; Sharma, R.; Kuila, A. Lipase production from Fusarium incarnatum KU377454 and its immobilization using Fe3O4 NPs for application in waste cooking oil degradation. Bioresour. Technol. Rep. 2019, 5, 134–140. [Google Scholar] [CrossRef]
- Geoffry, K.; Achur, R.N. Optimization of novel halophilic lipase production by Fusarium solani strain NFCCL 4084 using palm oil mill effluent. J. Genet. Eng. Biotechnol. 2018, 16, 327–334. [Google Scholar] [CrossRef]
- Żymanczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a versatile tool in biotechnology. In Yeast-Industrial Applications; Morata, A., Loira, I., Eds.; InTech: London, UK, 2017; pp. 3–40. [Google Scholar]
- Zhang, K.; Jin, Z.; Wang, P.; Zheng, S.P.; Han, S.Y.; Lin, Y. Improving the catalytic characteristics of lipase-displaying yeast cells by hydrophobic modification. Bioprocess Biosyst. Eng. 2017, 40, 1689–1699. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Zhao, G.; Wu, H.; Yu, Y.; Lai, F.; Xiao, X. Highly efficient synthesis of arbutin esters catalyzed by whole cells of Candida parapsilosis. RSC Adv. 2018, 8, 10081–10088. [Google Scholar] [CrossRef] [Green Version]
- Srimhan, P.; Kongnum, K.; Taweerodjanakarn, S.; Hongpattarakere, T. Selection of lipase producing yeasts for methanol-tolerant biocatalyst as whole cell application for palm-oil transesterification. Enzyme Microb. Technol. 2011, 48, 293–298. [Google Scholar] [CrossRef]
- Nuylert, A.; Hongpattarakere, T. Improvement of cell-bound lipase from Rhodotorula mucilaginosa P11I89 for use as a methanol-tolerant, whole-cell biocatalyst for production of palm-oil biodiesel. Ann. Microbiol. 2013, 63, 929–939. [Google Scholar] [CrossRef]
- Srimhan, P.; Hongpattarakere, T. Production of methanol tolerant cell-bound lipase of Magnusiomyces spicifer SPB2 and application as whole-cell biocatalyst in transesterification reaction. Trends Sci. 2023; in press. [Google Scholar]
- Louhasakul, Y.; Cheirsilp, B.; Prasertsan, P. Valorization of palm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valor. 2016, 7, 417–426. [Google Scholar] [CrossRef]
- Kuncharoen, N.; Techo, S.; Savarajara, A.; Tanasupawat, S. Identification and lipolytic activity of yeasts isolated from foods and wastes. Mycology 2020, 11, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, V.; Fonseca, C.; Lopes da Silva, T.; Roseiro, J.C.; Eusébio, A. Isolation and identification of Magnusiomyces capitatus as a lipase-producing yeast from olive mill wastewater. Waste Biomass Valor. 2020, 11, 3207–3221. [Google Scholar] [CrossRef] [Green Version]
- Baloch, K.A.; Upaichit, A.; Cheirsilp, B.; Fibriana, F. The occurrence of triple catalytic characteristics of yeast lipases and their application prospects in biodiesel production from non-edible Jatropha curcas oil in a solvent-free system. Curr. Microbiol. 2021, 78, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Niaz, M.; Iftikhar, T.; Qureshi, F.F.; Niaz, M. Extracellular lipase production by Aspergillus nidulans (Mbl-S-6) under submerged fermentation. Int. J. Agric. Biol. 2014, 16, 536–542. [Google Scholar]
- Aal, R.A.; Shetaia, Y.M.; Shafei, M.S.; Gomaa, S.K.; Menoufy, H.A.; Ei-Refai, H.A. Optimization of parameters for lipase production by Aspergillus niger NRRL-599 using response surface methodology. Egypt. Pharm. J. 2021, 18, 165–171. [Google Scholar]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Effect of process parameters on lipase production by Candida cylindracea in stirred tank bioreactor using renewable palm oil mill effluent based medium. J. Mol. Catal. B Enzym. 2011, 72, 187–192. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Potumarthi, R.; Subhakar, C.; Vanajakshi, J.; Jetty, A. Effect of aeration and agitation regimes on lipase production by newly isolated Rhodotorula mucilaginosa-MTCC 8737 in stirred tank reactor using molasses as sole production medium. Appl. Biochem. Biotechnol. 2008, 151, 700–710. [Google Scholar] [CrossRef]
- Colin, V.L.; Baigori, M.D.; Pera, L.M. Effect of environmental conditions on extracellular lipases production and fungal morphology from Aspergillus niger MYA 135. J. Basic Microbiol. 2010, 50, 52–58. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, A.F.; Taulk-Tornisielo, S.M.; Carmona, E.C. Influence of carbon and nitrogen sources on lipase production by a newly isolated Candida viswanathii strain. Ann. Microbiol. 2013, 63, 1225–1234. [Google Scholar] [CrossRef]
- Cesário, L.M.; Pires, G.P.; Pereira, R.F.S.; Fantuzzi, E.; da Silva Xavier, A.; Cassini, S.T.A.; de Oliveira, J.P. Optimization of lipase production using fungal isolates from oily residues. BMC Biotechnol. 2021, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.R.; Aguiar-Oliveira, E.; Pozza, E.L.; Costa, F.A.A.; Mazutti, M.A.; Maugeri, F.; Rodrigues, M.I. Application of yeast hydrolysate in extracellular lipase production by Geotrichum candidum in shaken flasks, stirred tank, and airlift reactors. Can. J. Chem. Eng. 2015, 93, 1524–1530. [Google Scholar] [CrossRef]
- Messias, J.M.; da Costa, B.Z.; de Lima, V.M.G.; Dekker, R.F.H.; Rezende, M.I.; Krieger, N.; Barbosa, A.M. Screening Botryosphaeria species for lipases: Production of lipase by Botryosphaeria ribis EC-01 grown on soybean oil and other carbon sources. Enzyme Microb. Technol. 2009, 45, 426–431. [Google Scholar] [CrossRef]
- Domínguez, A.; Deive, F.J.; Sanromán, M.A.; Longo, M.A. Effect of lipids and surfactants on extracellular lipase production by Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2003, 78, 1166–1170. [Google Scholar] [CrossRef]
- Tiss, A.; Carrière, F.; Douchet, I.; Patkar, S.; Svendsen, A.E.; Verger, R. Interfacial binding and activity of lipases at the lipid-water interface: Effects of gum arabic and surface pressure. Colloids Surf. B Biointerfaces 2002, 26, 135–145. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef] [Green Version]
- Brozzoli, V.; Crognale, S.; Sampedro, I.; Federici, F.; D’Annibale, A.; Petruccioli, M. Assessment of olive-mill wastewater as a growth medium for lipase production by Candida cylindracea in bench-top reactor. Bioresour. Technol. 2009, 100, 3395–3402. [Google Scholar] [CrossRef]
- Ahmed, A.; Badar, R.; Khalique, N. Screening and optimization of submerged fermentation of lipolytic Aspergillus oryzae. Bioresources 2019, 14, 7664–7674. [Google Scholar] [CrossRef]
- Domínguez De María, P.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Understanding Candida rugosa lipases: An overview. Biotechnol. Adv. 2006, 24, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Almoselhy, R.I.M. High-speed and high-pressure homogenization techniques for optimization of food processing, quality, and safety. J. Microb. Biotechnol. 2022, 7, 000243. [Google Scholar]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srimhan, P.; Hongpattarakere, T. Scale-Up Lipase Production and Development of Methanol Tolerant Whole-Cell Biocatalyst from Magnusiomyces spicifer SPB2 in Stirred-Tank Bioreactor and Its Application for Biodiesel Production. Catalysts 2023, 13, 617. https://doi.org/10.3390/catal13030617
Srimhan P, Hongpattarakere T. Scale-Up Lipase Production and Development of Methanol Tolerant Whole-Cell Biocatalyst from Magnusiomyces spicifer SPB2 in Stirred-Tank Bioreactor and Its Application for Biodiesel Production. Catalysts. 2023; 13(3):617. https://doi.org/10.3390/catal13030617
Chicago/Turabian StyleSrimhan, Purimprat, and Tipparat Hongpattarakere. 2023. "Scale-Up Lipase Production and Development of Methanol Tolerant Whole-Cell Biocatalyst from Magnusiomyces spicifer SPB2 in Stirred-Tank Bioreactor and Its Application for Biodiesel Production" Catalysts 13, no. 3: 617. https://doi.org/10.3390/catal13030617