Titanium Carbide Composite Hollow Cobalt Sulfide Heterojunction with Function of Promoting Electron Migration for Efficiency Photo-Assisted Electro-Fenton Cathode
Abstract
:1. Introduction
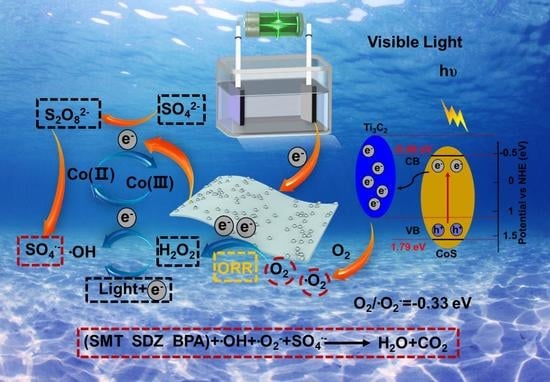
2. Results
3. Experimental Section
3.1. Chemicals
3.2. Preparation of CoS2/CoS and CoS2/CoS/Ti3C2
3.3. Characterization and Experimental Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.; Shen, C.; Zheng, H.; Liu, F.; Li, A. OCNTs encapsulating Fe-Co PBA as efficient chainmail-like electrocatalyst for enhanced heterogeneous electro-Fenton reaction. Appl. Catal. B. 2020, 269, 118–785. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B 2019, 255, 117–739. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong, L.T.X.; Bechelany, M.; Esposito, G.; van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Haider, M.R.; Jiang, W.L.; Han, J.L.; Sharif, H.M.A.; Ding, Y.C.; Cheng, H.Y.; Wang, A.J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B 2019, 256, 117–774. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Wen, R.; Zhang, H.; Elumalai, P.; Zheng, Q.; Chen, H.; Yang, Y.; Huang, M.; Ying, G. Heterogeneous electro-Fenton using three-dimension NZVI-BC electrodes for degradation of neonicotinoid wastewater. Water Res. 2020, 182, 115975. [Google Scholar] [CrossRef]
- Chu, L.; Sun, Z.; Fang, G.; Cang, L.; Wang, X.; Zhou, D.; Gao, J. Highly effective removal of BPA with boron-doped graphene shell wrapped FeS2 nanoparticles in electro-Fenton process: Performance and mechanism. Sep. Purif. Technol. 2021, 267, 118–680. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, K.; Quan, X.; Cao, P.; Chen, S.H. Highly efficient metal-free electro-Fenton degradation of organic contaminants on a bifunctional catalyst. J. Hazard. Mater. 2021, 416, 125859. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, H.; Shen, C.; Jiang, B.; Liu, F.; Li, A. Hierarchical Iron Phosphides Composite Confined in Ultrathin Carbon Layer as Effective Heterogeneous Electro-Fenton Catalyst with Prominent Stability and Catalytic Activity. Adv. Funct. Mater. 2021, 31, 2106311. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhang, J.; Liu, X.; Tao, S. Electronic structure engineering through Fe-doping CoP enables hydrogen evolution coupled with electro-Fenton. Nano Energy 2021, 84, 105–943. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Wang, Z.; Pang, H.; Li, L.; Chen, Y.; Zheng, Y.; Zhang, J. Mineralization of norfloxacin in a CoFe–LDH/CF cathode-based heterogeneous electro-fenton system: Preparation parameter optimization of the cathode and conversion mechanisms of H2O2 to ·OH. Chem. Eng. J. 2021, 417, 129–240. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, K.; Tang, Y.; Wei, Y.; Tang, H.; Du, Y.; Liu, H.; Chen, Y.; Liu, C. Hollow sea-urchin-shaped carbon-anchored single-atom iron as dual-functional electro-Fenton catalysts for degrading refractory thiamphenicol with fast reaction kinetics in a wide pH range. Chem. Eng. J. 2022, 427, 130–996. [Google Scholar] [CrossRef]
- Dong, P.; Chen, X.; Guo, M.; Wu, Z.; Wang, H.; Lin, F.; Zhang, J.; Wang, S.; Sun, C.; Zhao, H. Heterogeneous electro-Fenton catalysis with self-supporting CFP@MnO2-Fe3O4/C cathode for shale gas fracturing flowback wastewater. J. Hazard. Mater. 2021, 412, 125–208. [Google Scholar] [CrossRef]
- Guo, M.; Lu, M.; Zhao, H.; Lin, F.; He, F.; Zhang, J.; Wang, S.; Dong, P.; Zhao, C. Efficient electro-Fenton catalysis by self-supported CFP@CoFe2O4 electrode. J. Hazard. Mater. 2022, 423, 127033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, Y.; Ma, N.; Zhao, X. Promoted photoelectrocatalytic degradation of BPA with peroxymonosulfate on a MnFe2O4 modified carbon paper cathode. Chem. Eng. J. 2020, 399, 125088. [Google Scholar] [CrossRef]
- Guo, M.; Qayum, A.; Dong, S.; Jiao, X.; Chen, D.; Wang, T. In situ conversion of metal (Ni, Co or Fe) foams into metal sulfide (Ni3S2, Co9S8 or FeS) foams with surface grown N-doped carbon nanotube arrays as efficient superaerophobic electrocatalysts for overall water splitting. J. Mater. Chem. A 2020, 8, 9239–9247. [Google Scholar] [CrossRef]
- Sheng, H.; Hermes, E.D.; Yang, X.; Ying, D.; Janes, A.N.; Li, W.; Schmidt, J.R. Electrocatalytic Production of H2O2 by Selective Oxygen Reduction Using Earth-Abundant Cobalt Pyrite (CoS2). ACS Catal. 2019, 9, 8433–8442. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.H.; Lee, J.H.; Kim, J. Unravelling lewis acidic and reductive characters of normal and inverse nickel-cobalt thiospinels in directing catalytic H2O2 cleavage. J. Hazard. Mater. 2020, 392, 122347. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, Y.; Peng, X.; Xiao, Y.; Huang, J. Micro-nano structured CoS: An efficient catalyst for peroxymonosulfate activation for removal of bisphenol A. Sep. Purif. Technol. 2020, 233, 116022. [Google Scholar] [CrossRef]
- Yein, W.T.; Wang, Q.; Wu, J.; Wu, X. Converting CoS-TEA hybrid compound to CoS defective ultrathin nanosheets and their enhanced photocatalytic property. J. Mol. Liq. 2018, 268, 273–283. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A novel Z-scheme Ag3VO4/BiVO4 heterojunction photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Catal. B 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Zhang, Y.; Li, Y.; Fan, L.; Li, F.T.; Li, X. In-situ construction of sequential heterostructured CoS/CdS/CuS for building “electron-welcome zone” to enhance solar-to-hydrogen conversion. Appl. Catal. B 2022, 300, 120–763. [Google Scholar] [CrossRef]
- Ma, D.; Hu, B.; Wu, W.; Liu, X.; Zai, J.; Shu, C.; Tadesse Tsega, T.; Chen, L.; Qian, X.; Liu, T.L. Highly active nanostructured CoS2/CoS heterojunction electrocatalysts for aqueous polysulfide/iodide redox flow batteries. Nat. Commun. 2019, 10, 3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wang, X.; Liu, X.; Ling, C.; Shen, W.; Zhang, L. Visible light promoted Fe3S4 Fenton oxidation of atrazine. Appl. Catal. B 2020, 277, 119–229. [Google Scholar] [CrossRef]
- Zhao, R.; Qian, Z.; Liu, Z.; Zhao, D.; Hui, X.; Jiang, G.; Wang, C.; Yin, L. Molecular-level heterostructures assembled from layered black phosphorene and Ti3C2 MXene as superior anodes for high-performance sodium ion batteries. Nano Energy 2019, 65, 104037. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Li, J.; Liu, H. Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B 2020, 267, 118–379. [Google Scholar] [CrossRef]
- Liao, Y.; Qian, J.; Xie, G.; Han, Q.; Dang, W.; Wang, Y.; Lv, L.; Zhao, S.; Luo, L.; Zhang, W.; et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B 2020, 273, 119054. [Google Scholar] [CrossRef]
- Ai, Z.; Shao, Y.; Chang, B.; Huang, B.; Wu, Y.; Hao, X. Effective orientation control of photogenerated carrier separation via rational design of a Ti3C2(TiO2)@CdS/MoS2 photocatalytic system. Appl. Catal. B 2019, 242, 202–208. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, F.; Zhang, B.; Xu, C.; Yang, W.; Li, Y.F. Fabrication of novel FeS2 NWs/Ti3C2 cathode for Photo-Electro-Fenton degradation of sulfamethazine. Chem. Eng. J. 2021, 426, 130–719. [Google Scholar]
- Xu, H.; Cao, J.; Shan, C.; Wang, B.; Xi, P.; Liu, W.; Tang, Y. MOF-Derived Hollow CoS Decorated with CeOx Nanoparticles for Boosting Oxygen Evolution Reaction Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2018, 57, 8654–8658. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Arghavan, S.M.A.; Allahyari, E.; Arghavan, F.S.; Othmani, A.; Nasseh, N. Adsorption of malachite green dye. onto almond peel waste: A study focusing on application of the ANN approach for optimization of the effect of environmental parameters. Biomass Convers. Biorefinery 2020, 273, 119054. [Google Scholar] [CrossRef]
- Nasseh, N.; Samadi, M.T.; Ghadirian, M.; Panahi, A.H.; Rezaie, A. Photo-catalytic degradation of tamoxifen by using a novel. synthesized magnetic nanocomposite of FeCl2@ac@ ZnO: A study on the pathway, modeling, and sensitivity analysis using artificial neural network (AAN). J. Environ. Chem. Eng. 2022, 10, 107450. [Google Scholar] [CrossRef]
- Rahimi, S.M.; Panahi, A.H.; Allahyari, E.; Nasseh, N. Breaking down of low-biodegradation Acid Red 206 dye using bentonite/Fe3O4/ZnO magnetic nanocomposite as a novel photo-catalyst in presence of UV light. Chem. Phys. Lett. 2022, 794, 139480. [Google Scholar] [CrossRef]
- Fenton, J.L.; Schaak, R.E. Structure-Selective Cation Exchange in the Synthesis of Zincblende MnS and CoS Nanocrystals. Angew. Chem. Int. Ed. Engl. 2017, 56, 6464–6467. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.R.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. [Google Scholar] [CrossRef]
- Kinner, T.; Bhandari, K.P.; Bastola, E.; Monahan, B.M.; Haugen, N.O.; Roland, P.J.; Bigioni, T.P.; Ellingson, R.J. Majority Carrier Type Control of Cobalt Iron Sulfide (CoxFe1–xS2) Pyrite Nanocrystals. J. Phys. Chem. C 2016, 120, 5706–5713. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Zhu, Z.; Cheng, F.; Srinivansan, M.; Adams, S.; Ramakrishna, S. Unique Cobalt Sulfide/Reduced Graphene Oxide Composite as an Anode for Sodium-Ion Batteries with Superior Rate Capability and Long Cycling Stability. Small 2016, 12, 1359–1368. [Google Scholar] [CrossRef]
- Yun, X.; Lu, T.; Zhou, R.; Lu, Z.; Li, J.; Zhu, Y. Heterostructured NiSe2/CoSe2 hollow microspheres as battery-type cathode for hybrid supercapacitors: Electrochemical kinetics and energy storage mechanism. Chem. Eng. J. 2021, 426, 131–328. [Google Scholar] [CrossRef]
- Hao, J.; Yang, W.; Peng, Z.; Zhang, C.; Huang, Z.; Shi, W. A nitrogen doping method for CoS2 electrocatalysts with enhanced water oxidation performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, A.L.; Xiao, W.; Chao, D.; Zhang, X.; Tiep, N.H.; Chen, S.; Wang, J.; Kang, X.; Ding, J.; et al. In Situ Grown Epitaxial Heterojunction Exhibits High-Performance Electrocatalytic Water Splitting. Adv. Mater. 2018, 30, e1705516. [Google Scholar] [CrossRef]
- Chu, L.; Sun, Z.; Cang, L.; Fang, G.; Wang, X.; Zhou, D.; Gao, J. A novel sulfite coupling electro-fenton reactions with ferrous sulfide cathode for anthracene degradation. Chem. Eng. J. 2020, 400, 125945. [Google Scholar] [CrossRef]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Kwon, S.; Zhang, F.; Stephen, B.; Kim, K.K.; Jung, R.; Kwon, S.; Chung, K.B. Photocatalytic improvement of Mn-adsorbed g-C3N4. Appl. Catal. B 2017, 206, 271–281. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.; Oturan, M.A. Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine. Appl. Catal. B. 2016, 199, 331–341. [Google Scholar] [CrossRef]
- Yang, F.; He, M.; Wu, T.F.; Hao, A.P.; Zhang, S.B.; Chen, Y.D.; Zhou, S.B.; Zhen, L.Y.; Wang, R.; Yuan, Z.L.; et al. Sulfadiazine oxidation by permanganate: Kinetics, mechanistic investigation and toxicity evaluation. Chem. Eng. J. 2018, 349, 56–65. [Google Scholar] [CrossRef]
- Chen, F.; Yang, F.; Liu, H.; Che, S.; Zhang, G.; Xu, C.; Li, Y. One-pot preparation of surface vulcanization Co-Fe bimetallic aerogel for efficient sulfadiazine degradation. Chem. Eng. J. 2022, 430, 132–904. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Ma, N.; Li, Z.; Wu, M.; Xu, D.; Zhang, S.; Feng, W.; Zhu, Y. Bi4O5Br2 nanosheets with vertical aligned facets for efficient visible-light-driven photodegradation of BPA. Appl. Catal. B. 2021, 286, 119–937. [Google Scholar] [CrossRef]
- Deng, F.; Li, S.; Zhou, M.; Zhu, Y.; Qiu, S.; Li, K.; Ma, F.; Jiang, J. A biochar modified nickel-foam cathode with iron-foam catalyst in electro-Fenton for sulfamerazine degradation. Appl. Catal. B 2019, 256, 117–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wu, P.; Duan, Y.; Zhou, L.; Lai, Y.; Li, S. Three-dimensional heterogeneous Electro-Fenton system with a novel catalytic particle electrode for Bisphenol A removal. J. Hazard. Mater. 2020, 393, 120448. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yang, L.M.; Chen, J.P.; Zheng, Y.M. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation. J. Hazard. Mater. 2019, 371, 576–585. [Google Scholar] [CrossRef]
- Liu, K.; Yu, M.; Wang, H.; Wang, J.; Liu, W.; Hoffmann, M. Multiphase Porous Electrochemical Catalysts Derived from Iron-Based Metal-Organic Framework Compounds. Environ. Sci. Technol. 2019, 53, 6474–6482. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Yuan, S.; Mao, X.; Hu, W.; Liao, P.; Tong, M.; Alshawabkeh, A.N. Electrocatalytic activity of Pd-loaded Ti/TiO2 nanotubes cathode for TCE reduction in groundwater. Water Res. 2013, 47, 3573–3582. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Yang, F.; Che, S.; Liu, H.; Chen, N.; Wu, Z.; Li, Y. Titanium Carbide Composite Hollow Cobalt Sulfide Heterojunction with Function of Promoting Electron Migration for Efficiency Photo-Assisted Electro-Fenton Cathode. Catalysts 2023, 13, 253. https://doi.org/10.3390/catal13020253
Chen F, Yang F, Che S, Liu H, Chen N, Wu Z, Li Y. Titanium Carbide Composite Hollow Cobalt Sulfide Heterojunction with Function of Promoting Electron Migration for Efficiency Photo-Assisted Electro-Fenton Cathode. Catalysts. 2023; 13(2):253. https://doi.org/10.3390/catal13020253
Chicago/Turabian StyleChen, Fengjiang, Fan Yang, Sai Che, Hongchen Liu, Neng Chen, Zhijie Wu, and Yongfeng Li. 2023. "Titanium Carbide Composite Hollow Cobalt Sulfide Heterojunction with Function of Promoting Electron Migration for Efficiency Photo-Assisted Electro-Fenton Cathode" Catalysts 13, no. 2: 253. https://doi.org/10.3390/catal13020253