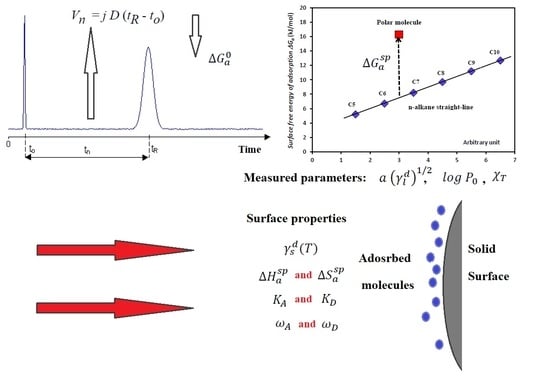
Effect of Modulation and Functionalization of UiO-66 Type MOFs on Their Surface Thermodynamic Properties and Lewis Acid–Base Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Powder X-ray Diffraction
2.2. Scanning Electron Microscopy
2.3. N2 Sorption–Desorption Measurements
2.4. Thermogravimetric Analysis
2.5. Effect of Modulation and Functionalization on the Dispersive Component of Surface Energy
2.6. Effect of Modulation and Functionalization on the Lewis Acid–Base Behavior
3. Materials and Methods
3.1. Materials and Synthesis Procedure
3.2. Structural Characterization and IGC Conditions
3.3. Models and Methods of Inverse Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to perfection: Ironing out the defects in metal–organic framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional mechanical stability of highly porous zirconium metal–organic framework UiO-66 and its important implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.; Wieme, J.; Vanduyfhuys, L.; Vandenbrande, S.; Maurin, G.; Verstraelen, T.; Waroquier, M.; Van Speybroek, V. Thermodynamic insight in the high-pressure behavior of UiO-66: Effect of linker defects and linker expansion. Chem. Mater. 2016, 28, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; Van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; et al. Synthesis modulation as a tool to increase the catalytic activity of metal–organic frameworks: The unique case of UiO-66 (Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922–11932. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, D. De facto methodologies toward the synthesis and scale-up production of UiO-66-type metal–organic frameworks and membrane materials. Dalton Trans. 2015, 44, 19018–19040. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect engineering: Tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Wan, W.; Zou, X.; Chater, P.A.; Kleppe, A.K.; Tucker, M.G.; Wilhelm, H.; Funnell, N.P.; Coudert, F.-X.; Goodwin, A.L. Correlated defect nanoregions in a metal–organic framework. Nat. Commun. 2014, 5, 4176. [Google Scholar] [CrossRef] [Green Version]
- Canivet, J.; Vandichel, M.; Farrusseng, D. Origin of highly active metal–organic framework catalysts: Defects? Defects! Dalton Trans. 2016, 45, 4090–4099. [Google Scholar] [CrossRef]
- Trickett, C.A.; Gagnon, K.J.; Lee, S.; Gándara, F.; Bürgi, H.-B.; Yaghi, O.M. Definitive molecular level characterization of defects in UiO-66 crystals. Angew. Chem. Int. Ed. 2015, 54, 11162–11167. [Google Scholar] [CrossRef]
- Xydias, P.; Spanopoulos, I.; Klontzas, E.; Froudakis, G.E.; Trikalitis, P.N. Drastic enhancement of the CO2 adsorption properties in sulfone-functionalized Zr-and Hf-UiO-67 MOFs with hierarchical mesopores. Inorg. Chem. 2014, 53, 679–681. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal–organic frameworks: From nano to single crystals. Chem.—A Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Zahn, G.; Zerner, P.; Lippke, J.; Kempf, F.L.; Lilienthal, S.; Schröder, C.A.; Schneider, A.M.; Behrens, P. Insight into the mechanism of modulated syntheses: In situ synchrotron diffraction studies on the formation of Zr-fumarate MOF. CrystEngComm 2014, 16, 9198–9207. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Rinsant, D. Elaboration de matériaux hybrides fonctionnalisés de type MOFs pour l’extraction sélective de l’uranium. Ph.D. Thesis, Université Montpellier, Montpellier, France, 2019. [Google Scholar]
- Jasuja, H.; Peterson, G.W.; Decoste, J.B.; Browe, M.A.; Walton, K.S. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air. Chem. Eng. Sci. 2015, 124, 118–124. [Google Scholar] [CrossRef]
- Peterson, G.W.; Mahle, J.J.; DeCoste, J.B.; Gordon, W.O.; Rossin, J.A. Extraordinary NO2 Removal by the Metal–Organic Framework UiO-66-NH2. Angew. Chem. 2016, 128, 6343–6346. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Tanabe, K.K.; Cohen, S.M. Functional group effects on metal–organic framework topology. Chem. Commun. 2012, 48, 9370–9372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal–organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.-Q.; Yuan, L.-Y.; Zhu, L.; Liu, Z.-R.; Chu, S.-Q.; Zheng, L.-R.; Zhang, J.; Chai, Z.-F.; Shi, W.-Q. Introduction of amino groups into acid-resistant MOFs for enhanced U (VI) sorption. J. Mater. Chem. A 2015, 3, 525–534. [Google Scholar] [CrossRef]
- Wang, L.L.; Luo, F.; Dang, L.L.; Li, J.Q.; Wu, X.L.; Liu, S.J.; Luo, M.B. Ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide-and carboxyl-functionalized metal–organic framework. J. Mater. Chem. A 2015, 3, 13724–13730. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Noh, H.; Ayoub, G.; Peterson, G.W.; Buru, C.T.; Islamoglu, T.; Farha, O.K. Scalable, room temperature, and water-based synthesis of functionalized zirconium-based metal–organic frameworks for toxic chemical removal. CrystEngComm 2019, 21, 2409–2415. [Google Scholar] [CrossRef]
- Das, A.; Das, S.; Trivedi, V.; Biswas, S. A dual functional MOF-based fluorescent sensor for intracellular phosphate and extracellular 4-nitrobenzaldehyde. Dalton Trans. 2019, 48, 1332–1343. [Google Scholar] [CrossRef]
- Molavi, H.; Eskandari, A.; Shojaei, A.; Mousavi, S.A. Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66 (Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2016, with Projections to 2040; Technical Report; Government Printing Office: Washington, DC, USA, 2016.
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Shearer, G.C.; Forselv, S.; Chavan, S.; Bordiga, S.; Mathisen, K.; Bjørgen, M.; Svelle, S.; Lillerud, K.P. In situ infrared spectroscopic and gravimetric characterisation of the solvent removal and dehydroxylation of the metal organic frameworks UiO-66 and UiO-67. Top. Catal. 2013, 56, 770–782. [Google Scholar] [CrossRef]
- Van de Voorde, B.; Stassen, I.; Bueken, B.; Vermoortele, F.; De Vos, D.; Ameloot, R.; Tan, J.-C.; Bennett, T.D. Improving the mechanical stability of zirconium-based metal–organic frameworks by incorporation of acidic modulators. J. Mater. Chem. A 2015, 3, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Ledwaba, M.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S.; Pang, W. Structural defects in metal–organic frameworks (MOFs): Formation, detection and control towards practices of interests. Coord. Chem. Rev. 2017, 349, 169–197. [Google Scholar] [CrossRef]
- Hu, Z.; Castano, I.; Wang, S.; Wang, Y.; Peng, Y.; Qian, Y.; Chi, C.; Wang, X.; Zhao, D. Modulator effects on the water-based synthesis of Zr/Hf metal–organic frameworks: Quantitative relationship studies between modulator, synthetic condition, and performance. Cryst. Growth Des. 2016, 16, 2295–2301. [Google Scholar] [CrossRef]
- Hamieh, T.; Ali-Ahmad, A.; Jrad, A.; Roques-Carmes, T.; Hmadeh, M.; Toufaily, J. Surface Thermodynamics and Lewis Acid-Base Properties of UiO-66 Crystals by Inverse Gas Chromatography at Infinite Dilution. SSRN, 2021; preprint. [Google Scholar] [CrossRef]
- Papirer, E.; Brendlé, E.; Balard, H.; Dentzer, J. Variation of the surface properties of nickel oxide upon heat treatment evidenced by temperature programmed desorption and inverse gas chromatography studies. J. Mater. Sci. 2000, 35, 3573–3577. [Google Scholar] [CrossRef]
- Balard, H.; Sidqi, M.; Papirer, E.; Donnet, J.B.; Tuel, A.; Hommel, H.; Legrand, A.P. Study of modified silicas by inverse gas chromatography part II: Influence of chain length on surface properties of silicas grafted with α−ω diols. Chromatographia 1988, 25, 712–716. [Google Scholar] [CrossRef]
- Jrad, A.; Hmadeh, M.; Tarboush, B.J.A.; Awada, G.; Ahmad, M. Structural engineering of Zr-based metal-organic framework catalysts for optimized biofuel additives production. Chem. Eng. J. 2020, 382, 122793. [Google Scholar] [CrossRef]
- Jrad, A.; Tarboush, B.J.A.; Hmadeh, M.; Ahmad, M. Tuning acidity in zirconium-based metal organic frameworks catalysts for enhanced production of butyl butyrate. Appl. Catal. A Gen. 2019, 570, 31–41. [Google Scholar] [CrossRef]
- Kemball, C.; Rideal, E.K. The adsorption of vapours on mercury. I. Non-polar substances. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1946, 187, 53–73. [Google Scholar]
- De Boer, J.H.; Kruyer, S. Entropy and mobility of adsorbed molecules: I. Procedure; atomic gases on charcoal. Proc. K. Ned. Akad. Van Wet. 1952, 55, 451–463. [Google Scholar]
- Hamieh, T. New methodology to study the dispersive component of the surface energy and acid–base properties of silica particles by inverse gas chromatography at infinite dilution. J. Chromatogr. Sci. 2022, 60, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Saint Flour, C.; Papirer, E. Gas-solid chromatography. A method of measuring surface free energy characteristics of short glass fibers. 1. Through adsorption isotherms. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 337–341. [Google Scholar] [CrossRef]
- Donnet, J.B.; Park, S.J.; Balard, H. Evaluation of specific interactions of solid surfaces by inverse gas chromatography. Chromatographia 1991, 31, 434–440. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A new topological index for molecular probes used in inverse gas chromatography for the surface nanorugosity evaluation. J. Colloid Interface Sci. 1997, 194, 207–216. [Google Scholar] [CrossRef]








| Number of Missing Linkers | Surface Area (m2/g) | Pore Volume (cc/g) | Particle Size (nm) | |
|---|---|---|---|---|
| UiO-66-00 | 1.2 | 886 | 0.53 | 142 |
| UiO-66-AA | 1.3 | 988 | 0.58 | 460 |
| UiO-66-FA | 1.75 | 1720 | 0.71 | 510 |
| UiO-66(NH2) | 1.56 | 703 | 0.48 | 207 |
| UiO-66-00 | |||
|---|---|---|---|
| Molecular Model | (mJ/m2) | (mJ·m−2·K−1) | (mJ/m2) |
| Cylindrical | = −0.067 T + 43.5 | −0.067 | 43.5 |
| Redlich−Kwong | = −0.130 T + 75.8 | −0.130 | 75.8 |
| VDW | = −0.080 T + 46.5 | −0.080 | 46.5 |
| Geometric | = −0.029 T + 26.5 | −0.029 | 26.5 |
| Spherical | = −0.307 T + 156.9 | −0.307 | 156.9 |
| Kiselev | = −0.080 T + 47.6 | −0.080 | 47.6 |
| Hamieh model | = −0.251 T + 113.4 | −0.251 | 113.4 |
| Dorris–Gray | = −0.117 T + 76.6 | −0.117 | 76.6 |
| Dorris–Gray–Hamieh | = −0.160 T + 88.4 | −0.160 | 88.4 |
| UiO-66-AA | |||
|---|---|---|---|
| Molecular Model | (mJ/m2) | (mJ·m−2·K−1) | (mJ/m2) |
| Cylindrical | = −0.163 T + 84.63 | −0.163 | 84.63 |
| Redlich−Kwong | = −0.286 T + 142.14 | −0.286 | 142.14 |
| VDW | = −0.175 T + 87.15 | −0.175 | 87.15 |
| Geometric | = −0.098 T + 56.71 | −0.098 | 56.71 |
| Spherical | = −0.601 T + 279.23 | −0.601 | 279.23 |
| Kiselev | = −0.180 T + 89.97 | −0.180 | 89.97 |
| Hamieh model | = −0.444 T + 190.86 | −0.444 | 190.86 |
| Dorris–Gray | = −0.139 T + 86.68 | −0.139 | 86.68 |
| Dorris–Gray–Hamieh | = −0.192 T + 112.2 | −0.192 | 112.2 |
| UiO-66-FA | |||
|---|---|---|---|
| Molecular Model | (mJ/m2) | (mJ·m−2·K−1) | (mJ/m2) |
| Cylindrical | = −0.21 T + 113.28 | −0.21 | 113.28 |
| Redlich−Kwong | = −0.37 T + 190.74 | −0.37 | 190.74 |
| VDW | = −0.23 T + 116.79 | −0.23 | 116.79 |
| Geometric | = −0.12 T + 74.801 | −0.12 | 74.80 |
| Spherical | = −0.81 T + 380.95 | −0.81 | 380.95 |
| Kiselev | = −0.40 T + 121.86 | −0.24 | 121.86 |
| Hamieh model | = −0.60 T + 260.91 | −0.60 | 260.91 |
| Dorris–Gray | = −0.19 T + 121.28 | −0.19 | 121.28 |
| Dorris–Gray–Hamieh | = −0.25 T + 138.16 | −0.25 | 138.16 |
| UiO-66(NH2) | |||
|---|---|---|---|
| Molecular Model | (mJ/m2) | (mJ·m−2·K−1) | (mJ/m2) |
| Cylindrical | = −0.3661 T + 239.31 | −0.3661 | 239.31 |
| Redlich–Kwong | = −0.7253 T + 423.68 | −0.7253 | 423.68 |
| VDW | = −0.4434 T + 259.24 | −0.4434 | 259.24 |
| Geometric | = −0.1638 T + 147.47 | −0.1638 | 147.47 |
| Spherical | = −1.7097 T + 876.13 | −1.7097 | 876.13 |
| Kiselev | = −0.4421 T + 262.67 | −0.4421 | 262.67 |
| Hamieh model | = −1.3896 T + 628.7 | −1.3896 | 628.7 |
| Dorris–Gray | = −0.209 T + 133.51 | −0.2090 | 133.51 |
| Dorris–Gray–Hamieh | = −0.807 T + 453.49 | −0.8070 | 453.49 |
| UiO-66-00 | ||||||
|---|---|---|---|---|---|---|
| Models and IGC Methods | KA | KD | KA/KD | 10−3·ωA | 10−3·ωD | ωA/ωD |
| Kiselev | 0.600 | 0.404 | 1.485 | 1.803 | 0.934 | 1.930 |
| Spherical | 0.581 | 0.473 | 1.230 | 0.473 | 0.284 | 1.666 |
| Geometric | 0.493 | 0.146 | 3.368 | 1.048 | 0.712 | 1.472 |
| Van der Waals | 1.014 | 0.190 | 5.345 | 2.200 | 1.835 | 1.199 |
| Redlich–Kwong | 0.772 | 0.469 | 1.646 | 2.302 | 1.436 | 1.603 |
| Cylindrical | 0.517 | 0.249 | 2.078 | 1.544 | 1.049 | 1.471 |
| Hamieh model | 0.490 | 0.292 | 1.678 | 1.745 | 0.977 | 1.786 |
| Topological index | 0.380 | 0.170 | 2.233 | 0.423 | 0.128 | 3.311 |
| Deformation polarizability | 0.412 | 0.204 | 2.021 | 0.351 | 0.098 | 3.579 |
| Vapor pressure | 0.357 | 0.336 | 1.063 | 0.259 | 0.106 | 2.440 |
| Average values | 0.56 | 0.29 | 1.915 | 1.21 | 0.76 | 1.607 |
| UiO-66-AA | ||||||
|---|---|---|---|---|---|---|
| Models and IGC Methods | KA | KD | KA/KD | 10−3·ωA | 10−3·ωD | ωA/ωD |
| Kiselev | 0.63 | 0.35 | 1.80 | 0.71 | 0.46 | 1.53 |
| Spherical | 0.59 | 0.41 | 1.43 | 0.93 | 0.62 | 1.49 |
| Geometric | 0.44 | 0.23 | 1.93 | 0.69 | 0.35 | 2.01 |
| Van der Waals | 0.49 | 0.26 | 1.91 | 0.73 | 0.44 | 1.66 |
| Redlich–Kwong | 0.48 | 0.27 | 1.80 | 0.96 | 0.64 | 1.50 |
| Cylindrical | 0.58 | 0.34 | 1.73 | 0.81 | 0.52 | 1.57 |
| Hamieh model | 0.57 | 0.18 | 3.14 | 0.52 | 0.35 | 1.48 |
| Topological index | 0.38 | 0.21 | 1.81 | 0.46 | 0.38 | 1.20 |
| Deformation polarizability | 0.50 | 0.30 | 1.67 | 0.69 | 0.09 | 7.63 |
| Vapor pressure | 0.32 | 0.16 | 1.96 | 0.38 | 0.31 | 1.24 |
| Average values | 0.50 | 0.27 | 1.84 | 0.69 | 0.42 | 1.65 |
| UiO-66-FA | ||||||
|---|---|---|---|---|---|---|
| Models and IGC Methods | KA | KD | KA/KD | 10−3·ωA | 10−3·ωD | ωA/ωD |
| Kiselev | 1.02 | 0.22 | 4.69 | 2.02 | 1.18 | 1.71 |
| Spherical | 1.12 | 0.25 | 4.50 | 2.28 | 1.03 | 2.23 |
| Geometric | 0.89 | 0.30 | 2.99 | 1.84 | 1.05 | 1.75 |
| Van der Waals | 0.96 | 0.44 | 2.20 | 2.20 | 1.06 | 2.09 |
| Redlich–Kwong | 1.00 | 0.49 | 2.03 | 2.05 | 1.18 | 1.74 |
| Cylindrical | 0.97 | 0.31 | 3.17 | 2.17 | 0.98 | 2.22 |
| Hamieh model | 1.09 | 0.39 | 2.79 | 2.24 | 1.04 | 2.14 |
| Topological index | 1.08 | 0.39 | 2.76 | 0.73 | 0.48 | 1.52 |
| Deformation polarizability | 1.12 | 0.41 | 2.72 | 0.98 | 0.51 | 1.92 |
| Vapor pressure | 1.26 | 0.42 | 3.04 | 1.06 | 0.47 | 2.26 |
| Average values | 1.05 | 0.36 | 2.91 | 1.76 | 0.90 | 1.96 |
| UiO-66(NH2) | ||||||
|---|---|---|---|---|---|---|
| Models and IGC Methods | KA | KD | KA/KD | 10−3·ωA | 10−3·ωD | ωA/ωD |
| Kiselev | 1.44 | 0.52 | 2.79 | 2.7 | 1.2 | 2.27 |
| Spherical | 1.45 | 0.78 | 1.85 | 3.7 | 1.1 | 3.44 |
| Geometric | 1.69 | 1.03 | 1.64 | 4.3 | 1.7 | 2.59 |
| Van der Waals | 1.56 | 0.82 | 1.89 | 1.6 | 0.2 | 7.79 |
| Redlich–Kwong | 1.62 | 0.95 | 1.70 | 2.1 | 0.51 | 4.23 |
| Cylindrical | 1.16 | 0.46 | 2.52 | 1.8 | 0.33 | 5.60 |
| Hamieh model | 1.06 | 0.45 | 2.35 | 1.0 | 0.38 | 2.73 |
| Topological index | 1.05 | 0.48 | 2.20 | 1.1 | 0.29 | 3.76 |
| Deformation polarizability | 0.90 | 0.45 | 2.62 | 2.3 | 0.7 | 3.26 |
| Vapor pressure | 0.70 | 0.5 | 2.75 | 2.7 | 1.2 | 2.27 |
| Average values | 1.26 | 0.61 | 2.07 | 3.7 | 1.1 | 3.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali-Ahmad, A.; Hamieh, T.; Roques-Carmes, T.; Hmadeh, M.; Toufaily, J. Effect of Modulation and Functionalization of UiO-66 Type MOFs on Their Surface Thermodynamic Properties and Lewis Acid–Base Behavior. Catalysts 2023, 13, 205. https://doi.org/10.3390/catal13010205
Ali-Ahmad A, Hamieh T, Roques-Carmes T, Hmadeh M, Toufaily J. Effect of Modulation and Functionalization of UiO-66 Type MOFs on Their Surface Thermodynamic Properties and Lewis Acid–Base Behavior. Catalysts. 2023; 13(1):205. https://doi.org/10.3390/catal13010205
Chicago/Turabian StyleAli-Ahmad, Ali, Tayssir Hamieh, Thibault Roques-Carmes, Mohamad Hmadeh, and Joumana Toufaily. 2023. "Effect of Modulation and Functionalization of UiO-66 Type MOFs on Their Surface Thermodynamic Properties and Lewis Acid–Base Behavior" Catalysts 13, no. 1: 205. https://doi.org/10.3390/catal13010205