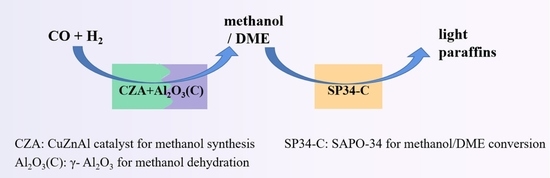
A Dual-Bed Strategy for Direct Conversion of Syngas to Light Paraffins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization Results
2.2. Catalytic Performance of Syngas to DME
2.3. Catalytic Conversion of Reaction Intermediates
2.4. Catalytic Performance of Syngas to Light Hydrocarbons
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Performance Evaluation
3.4. Thermodynamic Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yerga, R.M.N. Catalysts for production and conversion of syngas. Catalysts 2021, 11, 752. [Google Scholar] [CrossRef]
- Zhai, P.; Li, Y.; Wang, M.; Liu, J.; Cao, Z.; Zhang, J.; Xu, Y.; Liu, X.; Li, Y.-W.; Zhu, Q.; et al. Development of direct conversion of syngas to unsaturated hydrocarbons based on Fischer-Tropsch route. Chem 2021, 7, 3027–3051. [Google Scholar] [CrossRef]
- Pan, X.; Jiao, F.; Miao, D.; Bao, X. Oxide–zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer–Tropsch synthesis. Chem. Rev. 2021, 121, 6588–6609. [Google Scholar] [CrossRef] [PubMed]
- Torres Galvis, H.M.; de Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Lu, S.; Yang, H.; Zhou, Z.; Zhong, L.; Li, S.; Gao, P.; Sun, Y. Effect of In2O3 particle size on CO2 hydrogenation to lower olefins over bifunctional catalysts. Chin. J. Catal. 2021, 42, 2038–2048. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Zhang, P.; Yang, L.; Liu, S.; Li, Z. A facile approach for fabricating highly active ZrCeZnOx in combination with SAPO-34 for the conversion of syngas into light olefins. Appl. Surf. Sci. 2021, 542, 148713. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Zhang, P.; Yang, L.; Yang, G.; Ma, P.; Li, Z. Highly active ternary oxide ZrCeZnOx combined with SAPO-34 zeolite for direct conversion of syngas into light olefins. Catal. Today 2020, 368, 118–125. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, F.; Yang, L.; Yang, G.; Liang, X.; Li, Z. Syngas to olefins over a CrMnGa/SAPO-34 bifunctional catalyst: Effect of Cr and Cr/Mn ratio. Ind. Eng. Chem. Res. 2021, 60, 13214–13222. [Google Scholar] [CrossRef]
- Wang, Y. A new horizontal in C1 chemistry: Highly selective conversion of syngas to light olefins by a novel OX-ZEO process. J. Energy Chem. 2016, 25, 169–170. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, W.; Kang, J.; He, S.; Shi, S.; Zhang, Q.; Pan, Y.; Wen, W.; Wang, Y. Bifunctional catalysts for one-Step conversion of syngas into aromatics with excellent selectivity and stability. Chem 2017, 3, 334–347. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, S.; Wang, Y.; Zhang, L.; Wang, Y.; Zhang, G.; Min, X.; Cheng, K.; Zhang, Q.; Kang, J.; et al. Selective conversion of syngas to aromatics over a Mo−ZrO2/H-ZSM-5 bifunctional catalyst. ChemCatChem 2019, 11, 1681–1688. [Google Scholar] [CrossRef]
- Feng, J.; Miao, D.; Ding, Y.; Jiao, F.; Pan, X.; Bao, X. Direct synthesis of isoparaffin-rich gasoline from syngas. ACS Energy Lett. 2022, 7, 1462–1468. [Google Scholar] [CrossRef]
- Lin, Y.-G.; Hsu, Y.-K.; Chen, S.-Y.; Chen, L.-C.; Chen, K.-H. O2 plasma-activated CuO-ZnO inverse opals as high-performance methanol microreformer. J. Mater. Chem. 2010, 20, 10611–10614. [Google Scholar] [CrossRef]
- van den Berg, R.; Prieto, G.; Korpershoek, G.; van der Wal, L.I.; van Bunningen, A.J.; Lægsgaard-Jørgensen, S.; de Jongh, P.E.; de Jong, K.P. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 2016, 7, 13057. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Lee, Y.; Park, M.-J.; Lee, W.B. Practical microkinetic modeling approach for methanol synthesis from syngas over a Cu-based catalyst. Ind. Eng. Chem. Res. 2019, 58, 8663–8673. [Google Scholar] [CrossRef]
- Bhan, A.; Iglesia, E. A link between reactivity and local structure in acid catalysis on zeolites. Acc. Chem. Res. 2008, 41, 559–567. [Google Scholar] [CrossRef]
- Aloise, A.; Marino, A.; Dalena, F.; Giorgianni, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Bonura, G.; Frusteri, F.; Giordano, G. Desilicated ZSM-5 zeolite: Catalytic performances assessment in methanol to DME dehydration. Microporous Mesoporous Mater. 2020, 302, 110198. [Google Scholar] [CrossRef]
- Bae, J.W.; Kang, S.-H.; Lee, Y.-J.; Jun, K.-W. Synthesis of DME from syngas on the bifunctional Cu–ZnO–Al2O3/Zr-modified ferrierite: Effect of Zr content. Appl. Catal. B 2009, 90, 426–435. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, Y.; Fang, X.; Zhu, W.; Liu, Z. Highly converting syngas to lower olefins over a dual-bed catalyst. J. Energy Chem. 2021, 58, 573–576. [Google Scholar] [CrossRef]
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent progress in methanol-to-olefins (MTO) catalysts. Adv. Mater. 2019, 31, 1902181. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, Z.; Tian, P.; Chen, Z.; Fu, Y.; Zhu, W.; Liu, Z. A dual-bed catalyst for producing ethylene and propylene from syngas. J. Energy Chem. 2022, 66, 190–194. [Google Scholar] [CrossRef]
- Arora, S.S.; Nieskens, D.L.S.; Malek, A.; Bhan, A. Lifetime improvement in methanol-to-olefins catalysis over chabazite materials by high-pressure H2 co-feeds. Nat. Catal. 2018, 1, 666–672. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Tian, P.; Wang, L.; Li, X.; Lin, S.; Guo, X.; Liu, Z. Achieving a superlong lifetime in the zeolite-catalyzed MTO reaction under high pressure: Synergistic effect of hydrogen and water. ACS Catal. 2019, 9, 3017–3025. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Hao, J.; Wang, Q. Heat transfer and mechanical properties of wood-plastic composites filled with flake graphite. Thermochim. Acta 2018, 664, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Z.; Wang, Y.; Deng, Y.; Li, J. Synthesis of small-sized SAPO-34 crystals with varying template combinations for the conversion of methanol to olefins. Catalysts 2018, 8, 570. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Wang, G. Effect of preparation method on the structure and catalytic performance of CuZnO catalyst for low temperature syngas hydrogenation in liquid phase. Catal. Lett. 2018, 148, 1462–1471. [Google Scholar] [CrossRef]
- Unutulmazsoy, Y.; Cancellieri, C.; Lin, L.; Jeurgens, L.P.H. Reduction of thermally grown single-phase CuO and Cu2O thin films by in-situ time-resolved XRD. Appl. Surf. Sci. 2022, 588, 152896. [Google Scholar] [CrossRef]
- Park, S.; Inagaki, S.; Kubota, Y. Selective formation of light olefins from dimethyl ether over MCM-68 modified with phosphate species. Catal. Today 2016, 265, 218–224. [Google Scholar] [CrossRef]
- Cheng, K.; Gu, B.; Liu, X.; Kang, J.; Zhang, Q.; Wang, Y. Direct and highly selective conversion of synthesis gas into lower olefins: Design of a bifunctional catalyst combining methanol synthesis and carbon–carbon coupling. Angew. Chem. Int. Ed. 2016, 55, 4725–4728. [Google Scholar] [CrossRef]
- Meng, F.; Liang, X.; Wang, L.; Yang, G.; Huang, X.; Li, Z. Rational design of SAPO-34 zeolite in bifunctional catalysts for syngas conversion into light olefins. Ind. Eng. Chem. Res. 2022, 61, 11397–11406. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]








| Samples | Stotal a (m2·g−1) | Vtotal b (cm3·g−1) | D c (nm) | Amounts of Desorbed CO d (mmol/g) |
|---|---|---|---|---|
| CZA | 68 | 0.19 | 9.4 | 0.10 |
| Al2O3(C) | 238 | 0.41 | 4.9 | – |
| SP34-C | 488 | 0.49 | 68.7 | – |
| Product | CH4 | C2–C40 | C2–C4= | C5+ | CO2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| C20 | C30 | C40 | C4= | C4= | C4= | ||||
| Selectivity (%) a | 7.2 | 7.3 | 75.4 | 6.2 | 1.1 | 1.0 | 0.8 | 1.0 | 32.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Meng, F.; Li, B.; Zhang, J.; Li, Z. A Dual-Bed Strategy for Direct Conversion of Syngas to Light Paraffins. Catalysts 2022, 12, 967. https://doi.org/10.3390/catal12090967
Wang L, Meng F, Li B, Zhang J, Li Z. A Dual-Bed Strategy for Direct Conversion of Syngas to Light Paraffins. Catalysts. 2022; 12(9):967. https://doi.org/10.3390/catal12090967
Chicago/Turabian StyleWang, Lina, Fanhui Meng, Baozhen Li, Jinghao Zhang, and Zhong Li. 2022. "A Dual-Bed Strategy for Direct Conversion of Syngas to Light Paraffins" Catalysts 12, no. 9: 967. https://doi.org/10.3390/catal12090967