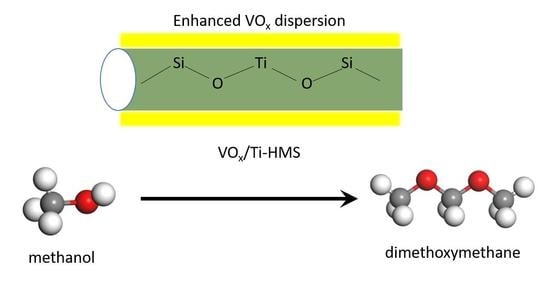
The Promoting Effect of Ti on the Catalytic Performance of V-Ti-HMS Catalysts in the Selective Oxidation of Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Supports and Catalysts
2.2. Confirmation of the Framework Ti
2.3. Effect of Ti on the Vanadia Dispersion
2.4. Redox Property and Catalytic Performance of Catalysts
3. Materials and Methods
3.1. Synthesis of the Supports
3.2. Preparation of the Catalysts
3.3. Characterization Methods
3.4. Catalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, W.; Chan, F.L.; Chaffee, A.L.; Wang, H.; Hoadley, A.; Tanksale, A. Dimethoxymethane Production via Catalytic Hydrogenation of Carbon Monoxide in Methanol Media. ACS Sustain. Chem. Eng. 2020, 8, 2081–2092. [Google Scholar] [CrossRef]
- Ftouni, K.; Lakiss, L.; Thomas, S.; Daturi, M.; Fernandez, C.; Bazin, P.; El Fallah, J.; El-Roz, M. TiO2/Zeolite Bifunctional (Photo)Catalysts for a Selective Conversion of Methanol to Dimethoxymethane: On the Role of Brønsted Acidity. J. Phys. Chem. C 2018, 122, 29359–29367. [Google Scholar] [CrossRef]
- Peláez, R.; Marín, P.; Ordóñez, S. Effect of formaldehyde precursor and water inhibition in dimethoxymethane synthesis from methanol over acidic ion exchange resins: Mechanism and kinetics. Biofuel Bioprod. Biorefin. 2021, 15, 1696–1708. [Google Scholar] [CrossRef]
- To, A.T.; Wilke, T.J.; Nelson, E.; Nash, C.P.; Bartling, A.; Wegener, E.C.; Unocic, K.A.; Habas, S.E.; Foust, T.D.; Ruddy, D.A. Dehydrogenative Coupling of Methanol for the Gas-Phase, One-Step Synthesis of Dimethoxymethane over Supported Copper Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 12151–12160. [Google Scholar] [CrossRef]
- Sun, R.; Delidovich, I.; Palkovits, R. Dimethoxymethane as a Cleaner Synthetic Fuel: Synthetic Methods, Catalysts, and Reaction Mechanism. ACS Catal. 2019, 9, 1298–1318. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.P.; Choi, H.; Kim, D.; Choi, M.; Ryoo, R.; Park, J.Y. The facet effect of ceria nanoparticles on platinum dispersion and catalytic activity of methanol partial oxidation. Chem. Commun. 2021, 57, 7382–7385. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Alavi, S.M. The effect of cesium and antimony promoters on the performance of Ti-phosphate-supported vanadium(V) oxide catalysts in selective oxidation of o-xylene to phthalic anhydride. Chem. Eng. Res. Des. 2015, 102, 286–296. [Google Scholar] [CrossRef]
- Wellmann, A.; Grazia, L.; Bermejo-Deval, R.; Sanchez-Sanchez, M.; Lercher, J.A. Effect of promoters on o-xylene oxidation pathways reveals nature of selective sites on TiO2 supported vanadia. J. Catal. 2022, 408, 330–338. [Google Scholar] [CrossRef]
- Dias, C.R.; Portela, M.F.; Bañares, M.A.; Galán-Fereres, M.; López-Granados, M.; Peña, M.A.; Fierro, J.L.G. Selective oxidation of o-xylene over ternary V-Ti-Si catalysts. Appl. Catal. A Gen. 2002, 224, 141–151. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Purdy, S.C.; He, Y.; Huang, Z.; You, R.; Wei, Z.; Meyer, H.M.; Yang, J.; Pan, Y.; et al. Multiple Promotional Effects of Vanadium Oxide on Boron Nitride for Oxidative Dehydrogenation of Propane. JACS Au 2022, 2, 1096–1104. [Google Scholar] [CrossRef]
- Kazerooni, H.; Towfighi Darian, J.; Mortazavi, Y.; Khadadadi, A.A.; Asadi, R. Titania-Supported Vanadium Oxide Synthesis by Atomic Layer Deposition and Its Application for Low-Temperature Oxidative Dehydrogenation of Propane. Catal. Lett. 2020, 150, 2807–2822. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.-G.; Xing, T.; Liu, Z.-T.; Liu, Z.-W.; Jiang, J.; Lu, J. Vanadium Oxide Supported on Titanosilicates for the Oxidative Dehydrogenation of n-Butane. Ind. Eng. Chem. Res. 2015, 54, 3602–3610. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X. The role of oxygen species in the selective oxidation of methanol to dimethoxymethane over VOx/TS-1 catalyst. J. Ind. Eng. Chem. 2017, 45, 296–300. [Google Scholar] [CrossRef]
- Wang, T.; Meng, Y.; Zeng, L.; Gong, J. Selective oxidation of methanol to dimethoxymethane over V2O5/TiO2–Al2O3 catalysts. Sci. Bull. 2015, 60, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Qin, Z.; Dong, M.; Zhu, H.; Wang, G.; Zhao, Y.; Fan, W.; Wang, J. Selective oxidation of methanol to dimethoxymethane over acid-modified V2O5/TiO2 catalysts. Fuel 2011, 90, 1335–1339. [Google Scholar] [CrossRef]
- Fan, Z.; Guo, H.; Fang, K.; Sun, Y. Efficient V2O5/TiO2 composite catalysts for dimethoxymethane synthesis from methanol selective oxidation. RSC Adv. 2015, 5, 24795–24802. [Google Scholar] [CrossRef]
- Mamedov, E.A.; Cortés Corberán, V. Oxidative dehydrogenation of lower alkanes on vanadium oxide-based catalysts. The present state of the art and outlooks. Appl. Catal. A Gen. 1995, 127, 1–40. [Google Scholar] [CrossRef]
- Khodakov, A.; Yang, J.; Su, S.; Iglesia, E.; Bell, A.T. Structure and properties of vanadium oxide-zirconia catalysts for propane oxidative dehydrogenation. J. Catal. 1998, 177, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Setnička, M.; Čičmanec, P.; Bulánek, R.; Zukal, A.; Pastva, J. Hexagonal mesoporous titanosilicates as support for vanadium oxide—Promising catalysts for the oxidative dehydrogenation of n-butane. Catal. Today 2013, 204, 132–139. [Google Scholar] [CrossRef]
- Santacesaria, E.; Cozzolino, M.; Di Serio, M.; Venezia, A.M.; Tesser, R. Vanadium based catalysts prepared by grafting: Preparation, properties and performances in the ODH of butane. Appl. Catal. A Gen. 2004, 270, 177–192. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, T.; Chen, S.; Zhao, Y.; Ma, X.; Gong, J. Selective oxidation of methanol to dimethoxymethane on V2O5–MoO3/γ-Al2O3 catalysts. Appl. Catal. B 2014, 160–161, 161–172. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Active component of supported vanadium catalysts in the selective oxidation of methanol. Kinet. Catal. 2016, 57, 82–94. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J.; Sauer, J. Support Effect in Oxide Catalysis: Methanol Oxidation on Vanadia/Ceria. J. Am. Chem. Soc. 2014, 136, 14616–14625. [Google Scholar] [CrossRef]
- Yoon, S.; Oh, K.; Liu, F.; Seo, J.H.; Somorjai, G.A.; Lee, J.H.; An, K. Specific metal–support interactions between nanoparticle layers for catalysts with enhanced methanol oxidation activity. ACS Catal. 2018, 8, 5391–5398. [Google Scholar] [CrossRef]
- Jaegers, N.R.; Wan, C.; Hu, M.Y.; Vasiliu, M.; Dixon, D.A.; Walter, E.; Wachs, I.E.; Wang, Y.; Hu, J.Z. Investigation of Silica-Supported Vanadium Oxide Catalysts by High-Field 51V Magic-Angle Spinning NMR. J. Phys. Chem. C 2017, 121, 6246–6254. [Google Scholar] [CrossRef]
- Castillo, R.; Koch, B.; Ruiz, P.; Delmon, B. Influence of the Amount of Titania on the Texture and Structure of Titania Supported on Silica. J. Catal. 1996, 161, 524–529. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Molecular Engineering of Supported Vanadium Oxide Catalysts Through Support Modification. Top. Catal. 2002, 18, 243–250. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Fierro, J.L.G.; Wachs, I.E. Structural Characteristics and Reactivity/Reducibility Properties of Dispersed and Bilayered V2O5/TiO2/SiO2 Catalysts. J. Phys. Chem. B 1999, 103, 618–629. [Google Scholar] [CrossRef]
- Mahipal Reddy, B.; Mehdi, S.; Padmanabha Reddy, E. Dispersion and thermal stability of vanadium oxide catalysts supported on titania-silica mixed oxide. Catal. Lett. 1993, 20, 317–327. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nayak, S.C.; Deo, G. TiO2/SiO2 supported vanadia catalysts for the ODH of propane. Catal. Today 2015, 254, 62–71. [Google Scholar] [CrossRef]
- Shee, D.; Mitra, B.; Chary, K.V.R.; Deo, G. Characterization and reactivity of vanadium oxide supported on TiO2-SiO2 mixed oxide support. Mol. Catal. 2018, 451, 228–237. [Google Scholar] [CrossRef]
- Hamilton, N.; Wolfram, T.; Tzolova Müller, G.; Hävecker, M.; Kröhnert, J.; Carrero, C.; Schomäcker, R.; Trunschke, A.; Schlögl, R. Topology of silica supported vanadium–titanium oxide catalysts for oxidative dehydrogenation of propane. Catal. Sci. Technol. 2012, 2, 1346–1359. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.H.; Herrera, J.E.; Hu, J.Z.; Wang, Y.; Peden, C.H.F. A new class of highly dispersed VOx catalysts on mesoporous silica: Synthesis, characterization, and catalytic activity in the partial oxidation of ethanol. Appl. Catal. A Gen. 2006, 300, 109–119. [Google Scholar] [CrossRef]
- Keränen, J.; Carniti, P.; Gervasini, A.; Iiskola, E.; Auroux, A.; Niinistö, L. Preparation by atomic layer deposition and characterization of active sites in nanodispersed vanadia/titania/silica catalysts. Catal. Today 2004, 91–92, 67–71. [Google Scholar] [CrossRef]
- Keränen, J.; Auroux, A.; Ek, S.; Niinistö, L. Preparation, characterization and activity testing of vanadia catalysts deposited onto silica and alumina supports by atomic layer deposition. Appl. Catal. A Gen. 2002, 228, 213–225. [Google Scholar] [CrossRef]
- Bérubé, F.; Kleitz, F.; Kaliaguine, S. Surface properties and epoxidation catalytic activity of Ti-SBA15 prepared by direct synthesis. J. Mater. Sci. 2009, 44, 6727–6735. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Xiu, J.; Han, X.; Bao, X. Direct synthesis, characterization and catalytic activity of titanium-substituted SBA-15 mesoporous molecular sieves. Appl. Catal. A Gen. 2004, 273, 185–191. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. Production of glycerol carbonate using a novel Ti-SBA-15 catalyst. Chem. Eng. J. 2018, 346, 477–488. [Google Scholar] [CrossRef]
- Bérubé, F.; Nohair, B.; Kleitz, F.; Kaliaguine, S. Controlled Postgrafting of Titanium Chelates for Improved Synthesis of Ti-SBA-15 Epoxidation Catalysts. Chem. Mater. 2010, 22, 1988–2000. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Pawelec, B.; Fierro, J.L.G.; Halachev, T. Removal of refractory S-containing compounds from liquid fuels on novel bifunctional CoMo/HMS catalysts modified with Ti. Appl. Catal. B 2007, 71, 223–236. [Google Scholar] [CrossRef]
- Düzenli, D.; Sahin, Ö.; Kazıcı, H.Ç.; Aktaş, N.; Kivrak, H. Synthesis and characterization of novel Ti doped hexagonal mesoporous silica catalyst for nonenzymatic hydrogen peroxide oxidation. Microporous Mesoporous Mater. 2018, 257, 92–98. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Infantes-Molina, A.; de Leon, J.N.D.; Obeso-Estrella, R.; Fuentes, S.; Alonso-Nuñez, G.; Pawelec, B. Synthesis and characterization of Ga-modified Ti-HMS oxide materials with varying Ga content. J. Mol. Catal. A Chem. 2015, 397, 26–35. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Ma, X.; Gong, J. Tuning Porosity of Ti-MCM-41: Implication for Shape Selective Catalysis. ACS Appl. Mater. Interfaces 2011, 3, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhao, D.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Room Temperature Synthesis of Ti–MCM-48 and Ti–MCM-41 Mesoporous Materials and Their Performance on Photocatalytic Splitting of Water. J. Phys. Chem. C 2012, 116, 1605–1613. [Google Scholar] [CrossRef]
- Song, H.; Wang, J.; Wang, Z.; Song, H.; Li, F.; Jin, Z. Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst. J. Catal. 2014, 311, 257–265. [Google Scholar] [CrossRef]
- Zhao, D.; Budhi, S.; Rodriguez, A.; Koodali, R.T. Rapid and facile synthesis of Ti-MCM-48 mesoporous material and the photocatalytic performance for hydrogen evolution. Int. J. Hydrog. Energy 2010, 35, 5276–5283. [Google Scholar] [CrossRef]
- Peng, R.; Wu, C.-M.; Baltrusaitis, J.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Ultra-stable CdS incorporated Ti-MCM-48 mesoporous materials for efficient photocatalytic decomposition of water under visible light illumination. Chem. Commun. 2013, 49, 3221–3223. [Google Scholar] [CrossRef]
- Huang, F.; Hao, H.; Sheng, W.; Dong, X.; Lang, X. Embedding an organic dye into Ti-MCM-48 for direct photocatalytic selective aerobic oxidation of sulfides driven by green light. Chem. Eng. J. 2022, 432, 134285. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. A Neutral Templating Route to Mesoporous Molecular Sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Fierro, J.L.G.; Pawelec, B.; Nava, R.; Klimova, T.; Fuentes, G.A.; Halachev, T. Synthesis and Characterization of Ti-HMS and CoMo/Ti-HMS Oxide Materials with Varying Ti Content. Chem. Mater. 2005, 17, 4062–4073. [Google Scholar] [CrossRef]
- Davis, R.J.; Liu, Z. Titania−Silica: A Model Binary Oxide Catalyst System. Chem. Mater. 1997, 9, 2311–2324. [Google Scholar] [CrossRef]
- Schraml-Marth, M.; Walther, K.L.; Wokaun, A.; Handy, B.E.; Baiker, A. Porous silica gels and TiO2/SiO2 mixed oxides prepared via the sol-gel process: Characterization by spectroscopic techniques. J. Non-Cryst. Solids 1992, 143, 93–111. [Google Scholar] [CrossRef]
- Boahene, P.E.; Soni, K.; Dalai, A.K.; Adjaye, J. Hydrotreating of coker light gas oil on Ti-modified HMS supports using Ni/HPMo catalysts. Appl. Catal. B 2011, 101, 294–305. [Google Scholar] [CrossRef]
- Soni, K.; Boahene, P.E.; Chandra Mouli, K.; Dalai, A.K.; Adjaye, J. Hydrotreating of coker light gas oil on Ti-HMS supported heteropolytungstic acid catalysts. Appl. Catal. A Gen. 2011, 398, 27–36. [Google Scholar] [CrossRef]
- Boccuti, M.R.; Rao, K.M.; Zecchina, A.; Leofanti, G.; Petrini, G. Spectroscopic Characterization of Silicalite and Titanium-Silicalite. In Studies in Surface Science and Catalysis; Morterra, C., Zecchina, A., Costa, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 48, pp. 133–144. [Google Scholar]
- Zhang, W.; Fröba, M.; Wang, J.; Tanev, P.T.; Wong, J.; Pinnavaia, T.J. Mesoporous Titanosilicate Molecular Sieves Prepared at Ambient Temperature by Electrostatic (S+I-, S+X-I+) and Neutral (S°I°) Assembly Pathways: A Comparison of Physical Properties and Catalytic Activity for Peroxide Oxidations. J. Am. Chem. Soc. 1996, 118, 9164–9171. [Google Scholar] [CrossRef]
- Bulánek, R.; Čapek, L.; Setnička, M.; Čičmanec, P. DR UV–vis Study of the Supported Vanadium Oxide Catalysts. J. Phys. Chem. C 2011, 115, 12430–12438. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Investigation of Surface Structures of Supported Vanadium Oxide Catalysts by UV−vis−NIR Diffuse Reflectance Spectroscopy. J. Phys. Chem. B 2000, 104, 1261–1268. [Google Scholar] [CrossRef]
- Tian, H.; Ross, E.I.; Wachs, I.E. Quantitative Determination of the Speciation of Surface Vanadium Oxides and Their Catalytic Activity. J. Phys. Chem. B 2006, 110, 9593–9600. [Google Scholar] [CrossRef]
- Magg, N.; Immaraporn, B.; Giorgi, J.B.; Schroeder, T.; Bäumer, M.; Döbler, J.; Wu, Z.; Kondratenko, E.; Cherian, M.; Baerns, M.; et al. Vibrational spectra of alumina- and silica-supported vanadia revisited: An experimental and theoretical model catalyst study. J. Catal. 2004, 226, 88–100. [Google Scholar] [CrossRef]
- Besselmann, S.; Freitag, C.; Hinrichsen, O.; Muhler, M. Temperature-programmed reduction and oxidation experiments with V2O5/TiO2 catalysts. Phys. Chem. Chem. Phys. 2001, 3, 4633–4638. [Google Scholar] [CrossRef]
- Kim, T.; Wachs, I.E. CH3OH oxidation over well-defined supported V2O5/Al2O3 catalysts: Influence of vanadium oxide loading and surface vanadium–oxygen functionalities. J. Catal. 2008, 255, 197–205. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Zemlyanov, D.Y.; Beloshapkin, S.A.; Knop-Gericke, A.; Schlögl, R.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst. J. Cata. 2014, 311, 59–70. [Google Scholar] [CrossRef] [Green Version]









| Sample | SBET a (m2/g) | VP b (cm3/g) | Dads c (nm) | Eg d (eV) |
|---|---|---|---|---|
| HMS | 764 | 0.67 | 3.71 | - |
| Ti-HMS(50) | 730 | 0.97 | 4.46 | - |
| Ti-HMS(30) | 662 | 0.93 | 5.22 | - |
| Ti-HMS(20) | 662 | 0.90 | 4.78 | - |
| Ti-HMS(10) | 661 | 0.59 | 3.80 | - |
| 28V-HMS | 382 | 0.45 | 4.68 | 2.47 |
| 28V-Ti-HMS(50) | 364 | 0.43 | 5.66 | 2.59 |
| 28V-Ti-HMS(30) | 329 | 0.38 | 5.22 | 2.73 |
| 28V-Ti-HMS(20) | 345 | 0.44 | 5.24 | 2.57 |
| 28V-Ti-HMS(10) | 343 | 0.39 | 4.84 | 2.55 |
| Sample | Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| DMM | MF | FA | DME | COx | ||
| 28V-HMS | 6 | 89 | 2 | 6 | 3 | 0 |
| 28V-Ti-HMS(50) | 12 | 85 | 7 | 6 | 2 | 0 |
| 28V-Ti-HMS(30) | 15 | 87 | 4 | 7 | 2 | 0 |
| 28V-Ti-HMS(20) | 11 | 84 | 5 | 8 | 3 | 0 |
| 28V-Ti-HMS(10) | 8 | 81 | 5 | 12 | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, L.B.; Kim, K.S.; Fu, J.; Chen, B. The Promoting Effect of Ti on the Catalytic Performance of V-Ti-HMS Catalysts in the Selective Oxidation of Methanol. Catalysts 2022, 12, 869. https://doi.org/10.3390/catal12080869
Sim LB, Kim KS, Fu J, Chen B. The Promoting Effect of Ti on the Catalytic Performance of V-Ti-HMS Catalysts in the Selective Oxidation of Methanol. Catalysts. 2022; 12(8):869. https://doi.org/10.3390/catal12080869
Chicago/Turabian StyleSim, Ley Boon, Kek Seong Kim, Jile Fu, and Binghui Chen. 2022. "The Promoting Effect of Ti on the Catalytic Performance of V-Ti-HMS Catalysts in the Selective Oxidation of Methanol" Catalysts 12, no. 8: 869. https://doi.org/10.3390/catal12080869