Kinetic Model of Catalytic Steam Gasification of 2-Methoxy-4-methylphenol Using 5% Ni–0.25% Ru/γAl2O3 in a CREC-Riser Simulator
Abstract
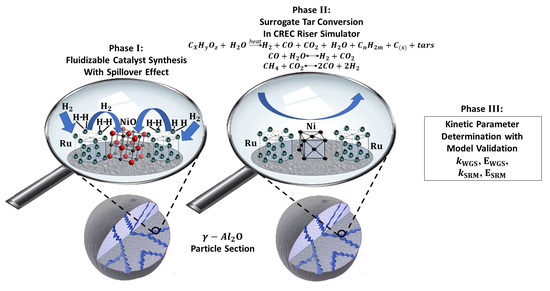
:1. Introduction
2. Results and Discussion
2.1. Spillover Effect from TPR Analysis
2.2. Experimental Gasification Results
3. Model Development
3.1. Water–Gas Shift Mechanism
3.2. Methane Steam Reforming Mechanism
3.3. Gasification in the CREC Riser Simulator
3.4. CO2 Adsorption Constant
3.5. Kinetic Parameters Calculations
4. Experimental Methods
4.1. Catalyst Preparation and Characterization
4.2. Temperature Programmed Reduction (TPR)
4.3. Biomass Gasification
4.4. CO2 Adsorption Constant
5. Conclusions
- A 5% Ni–0.25% Ru/γAl2O3 fluidizable catalyst with intrinsic H2 spillover ability can be successfully used for 2M4MP gasification.
- The performance of this 5% Ni–0.25% Ru/γAl2O3 catalyst can be described using a comprehensive kinetics.
- This kinetic model can be set as the addition of water–gas shift and steam methane reforming reactions.
- This postulated kinetics can be established neglecting char gasification given the minimal amounts of coke-on-catalyst observed.
- The kinetic parameter estimations can benefit from the uncoupling of intrinsic kinetics and CO2 adsorption, yielding parameters with narrow 95% confidence intervals and low parameter cross-correlation.
- The energies of activation obtained are in line with those reported in the technical literature, while the pre-exponential factors reflect the significantly enhanced activity of the 5% Ni–0.25% Ru/γAl2O3 catalyst.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dafedar, A.A.; Verma, S.S.; Yadav, A. Hydrogen Storage Techniques for Stationary and Mobile Applications: A Review. In Proceedings of the Recent Advances in Sustainable Technologies; Jha, K., Gulati, P., Tripathi, U.K., Eds.; Springer: Singapore, 2021; pp. 29–40. [Google Scholar]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Li, Z.; Chen, D. Characteristics of Tar Formation during Cellulose, Hemicellulose and Lignin Gasification. Fuel 2014, 118, 250–256. [Google Scholar] [CrossRef]
- Salaices, E.; Serrano, B.; de Lasa, H. Biomass Catalytic Steam Gasification Thermodynamics Analysis and Reaction Experiments in a CREC Riser Simulator. Ind. Eng. Chem. Res. 2010, 49, 6834–6844. [Google Scholar] [CrossRef]
- Mazumder, J.; de Lasa, H.I. Catalytic Steam Gasification of Biomass Surrogates: Thermodynamics and Effect of Operating Conditions. Chem. Eng. J. 2016, 293, 232–242. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Wang, Y. Catalysts for Steam Reforming of Bio-Oil: A Review. Ind. Eng. Chem. Res. 2017, 56, 4627–4637. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Coke Formation and Deactivation during Catalytic Reforming of Biomass and Waste Pyrolysis Products: A Review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]
- Calzada Hernandez, A.R.; Gibran González Castañeda, D.; Sánchez Enriquez, A.; de Lasa, H.; Serrano Rosales, B. Ru-Promoted Ni/γAl2O3 Fluidized Catalyst for Biomass Gasification. Catalysts 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Yang, F.-L.; Wei, X.-Y. Recent Advances in Syngas Production from Biomass Catalytic Gasification: A Critical Review on Reactors, Catalysts, Catalytic Mechanisms and Mathematical Models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Sharma, S.; Hines, L. Oxidation of Ruthenium. IEEE Trans. Compon. Hybrids Manuf. Technol. 1983, 6, 89–92. [Google Scholar] [CrossRef]
- Katheria, S.; Deo, G.; Kunzru, D. Rh-Ni/MgAl2O4 Catalyst for Steam Reforming of Methane: Effect of Rh Doping, Calcination Temperature and Its Application on Metal Monoliths. Appl. Catal. A Gen. 2019, 570, 308–318. [Google Scholar] [CrossRef]
- Kosukegawa, T.; Takao, T. Synthesis of a Heterometallic Spiked Tetrahedral Cluster of Ruthenium and Nickel Containing Multiple Hydrido Ligands and Its Degradation to a Tetrahedral NiRu3 Cluster. J. Organomet. Chem. 2019, 882, 70–79. [Google Scholar] [CrossRef]
- Sermon, P.A.; Bond, G.C. Hydrogen Spillover. Catal. Rev. 1974, 8, 211–239. [Google Scholar] [CrossRef]
- Mazumder, J.; de Lasa, H.I. Fluidizable La2O3 Promoted Ni/γ-Al2O3 Catalyst for Steam Gasification of Biomass: Effect of Catalyst Preparation Conditions. Appl. Catal. B Environ. 2015, 168–169, 250–265. [Google Scholar] [CrossRef]
- Atias, J.A.; de Lasa, H. Adsorption and Catalytic Reaction in FCC Catalysts Using a Novel Fluidized CREC Riser Simulator. Chem. Eng. Sci. 2004, 59, 5663–5669. [Google Scholar] [CrossRef]
- Escobedo, S.; Serrano, B.; Calzada, A.; Moreira, J.; de Lasa, H. Hydrogen Production Using a Platinum Modified TiO2 Photocatalyst and an Organic Scavenger. Kinetic Modeling. Fuel 2016, 181, 438–449. [Google Scholar] [CrossRef]
- Fogler, H.S.; Fogler, S.H. Elements of Chemical Reaction Engineering; Pearson Educación: London, UK, 1999; ISBN 978-970-26-0079-4. [Google Scholar]
- Mazumder, J.; de Lasa, H.I. Steam Gasification of a Cellulosic Biomass Surrogate Using a Ni/La2O3-ΓAl2O3 Catalyst in a CREC Fluidized Riser Simulator. Kinetics and Model Validation. Fuel 2018, 216, 101–109. [Google Scholar] [CrossRef]
- Salaices, E.; de Lasa, H.; Serrano, B. Steam Gasification of a Cellulose Surrogate over a Fluidizable Ni/α-Alumina Catalyst: A Kinetic Model. AIChE J. 2012, 58, 1588–1599. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: I. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Mark, M.F.; Maier, W.F.; Mark, F. Reaction Kinetics of the CO2 Reforming of Methane. Chem. Eng. Technol. 1997, 20, 361–370. [Google Scholar] [CrossRef]
- Maestri, M.; Vlachos, D.G.; Beretta, A.; Groppi, G.; Tronconi, E. Steam and Dry Reforming of Methane on Rh: Microkinetic Analysis and Hierarchy of Kinetic Models. J. Catal. 2008, 259, 211–222. [Google Scholar] [CrossRef]
- Spencer, M.S. On the Activation Energies of the Forward and Reverse Water-Gas Shift Reaction. Catal. Lett. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Ahmed, K.; Foger, K. Kinetics of Internal Steam Reforming of Methane on Ni/YSZ-Based Anodes for Solid Oxide Fuel Cells. Catal. Today 2000, 63, 479–487. [Google Scholar] [CrossRef]
- Jarosch, K.; El Solh, T.; de Lasa, H.I. Modelling the Catalytic Steam Reforming of Methane: Discrimination between Kinetic Expressions Using Sequentially Designed Experiments. Chem. Eng. Sci. 2002, 57, 3439–3451. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Reactant-Promoted Reaction Mechanism for Water-Gas Shift Reaction on Rh-Doped CeO2. J. Catal. 1993, 141, 71–81. [Google Scholar] [CrossRef]
- Matus, L.; Hozer, Z.; Toth, B.; Ver, N.; Kunstar, M.; Pinter, A.; Osan, J. Oxidation and Release of Ruthenium from White Inclusions; EUR 22730 EN. JRC36911; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Rostom, S. Propane Oxidative Dehydrogenation Under Oxygen-Free Conditions Using Novel Fluidizable Catalysts: Reactivity, Kinetic Modeling and Simulation Study; Electronic Thesis and Dissertation Repository; Western Libraries: London, ON, Canada, 2018. [Google Scholar]




| Catalyst | Decomposition Temperatures of Ni(NO3)2 (°C) | Reduction Temperatures (°C) | |
|---|---|---|---|
| Ni | Ru | ||
| Cat A: 5%Ni/γAl2O3 | 303 | 488 | --- |
| Cat B: 5%Ni-0.25%Ru/γAl2O3 | 268 | 400 | 245 |
| Cat C: 5%Ni-0.5%Ru/γAl2O3 | 252 | 390 | 223 |
| Cat D: 5%Ni-1.0%Ru/γAl2O3 | 248 | 380 | 210 |
| Reactions | ||
|---|---|---|
| Decomposition of nickel nitrate. | (1) | |
| Reduction of nickel oxide. | (2) | |
| Formation of ruthenium oxide. | (3) | |
| Reduction of ruthenium oxide. | (4) | |
| Catalysts | H2 Consumption (cm3/g STP) for NiO | Reducible Ni (wt %) | H2 Consumption (cm3/g STP) for RuO3 | Reducible Ru (wt %) |
|---|---|---|---|---|
| Cat A: 5% Ni/γAl2O3 | 7.73 | 2.02 | ||
| Cat B: 5% Ni–0.25% Ru/γAl2O3 | 10.12 | 2.71 | 2.2 | 0.33 |
| Cat C: 5% Ni–0.5% Ru/γAl2O3 | 12.07 | 3.16 | 3.21 | 0.48 |
| Cat D: 5% Ni–1.0% Ru/γAl2O3 | 14.86 | 3.89 | 4.13 | 0.77 |
| Time (s) | Partial Pressure (atm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 550 °C | 600 °C | 650 °C | |||||||||||||
| H2 | CO | CO2 | CH4 | H2O | H2 | CO | CO2 | CH4 | H2O | H2 | CO | CO2 | CH4 | H2O | |
| 5 | 0.24 | 0.27 | 0.6 | 0.27 | 1.42 | 0.46 | 0.33 | 0.36 | 0.21 | 1.51 | 0.63 | 0.42 | 0.43 | 0.23 | 1.59 |
| 10 | 0.42 | 0.21 | 0.62 | 0.21 | 1.38 | 0.7 | 0.29 | 0.47 | 0.2 | 1.4 | 0.78 | 0.42 | 0.44 | 0.2 | 1.52 |
| 15 | 0.46 | 0.2 | 0.65 | 0.15 | 1.33 | 0.8 | 0.28 | 0.53 | 0.18 | 1.3 | 0.85 | 0.4 | 0.45 | 0.17 | 1.37 |
| 20 | 0.49 | 0.17 | 0.67 | 0.13 | 1.29 | 0.9 | 0.27 | 0.53 | 0.15 | 1.25 | 1.09 | 0.37 | 0.57 | 0.14 | 1.34 |
| 30 | 0.49 | 0.09 | 0.73 | 0.12 | 1.25 | 0.97 | 0.24 | 0.59 | 0.14 | 1.17 | 1.2 | 0.35 | 0.59 | 0.13 | 1.31 |
| Water Gas Shift (WGS) | (7) | |
| Steam reforming of methane (SRM) | (8) | |
| Dry reforming of methane (DRM) | (9) | |
| Char gasificaton | (10) | |
| Boudouard gasification | (11) | |
| Hydrogenating gasification | (12) |
| Parameter | Value |
|---|---|
| (1/atm) | 0.49 |
| (kJ/mol) | −35.63 |
| Kinetic Constants | Value |
|---|---|
| (mol gcat−1 s−1 atm−2) | 4.5 × 10−4 ± 3.1 × 10−5 |
| (mol gcat−1 s−1 atm−2) | 1.16 × 10−3 ± 2.3 × 10−4 |
| Parameters | Catalyst 5% Ni–0.25% Ru/γAl2O3 | Mazumder et al. [18] * 20% Ni/5% La2O3-γAl2O3 | Salaices et al. [19] 2.5% Ni–αAl2O3 | |
|---|---|---|---|---|
| Value | 95% Confidence Intervals | Value | Value | |
(mol gcat−1 s−1 bar−2) | 4.1 × 10−4 | ±2.3 × 10−5 | 6.1 × 10−5 | 1.0 × 10−6 |
| EWGS (kJ/mol) | 20.2 | ± 5.3 | 33.3 | 23.4 |
(mol gcat−1 s−1 bar−2) | 2.5 × 10−3 | ±6.8 × 10−4 | 1.1 × 10−4 | 2.4 × 10−9 |
| ESRM (kJ/mol) | 63.9 | ± 7.1 | 68.1 | 32.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzada Hernandez, A.R.; Serrano Rosales, B.; de Lasa, H. Kinetic Model of Catalytic Steam Gasification of 2-Methoxy-4-methylphenol Using 5% Ni–0.25% Ru/γAl2O3 in a CREC-Riser Simulator. Catalysts 2022, 12, 282. https://doi.org/10.3390/catal12030282
Calzada Hernandez AR, Serrano Rosales B, de Lasa H. Kinetic Model of Catalytic Steam Gasification of 2-Methoxy-4-methylphenol Using 5% Ni–0.25% Ru/γAl2O3 in a CREC-Riser Simulator. Catalysts. 2022; 12(3):282. https://doi.org/10.3390/catal12030282
Chicago/Turabian StyleCalzada Hernandez, Alán Rubén, Benito Serrano Rosales, and Hugo de Lasa. 2022. "Kinetic Model of Catalytic Steam Gasification of 2-Methoxy-4-methylphenol Using 5% Ni–0.25% Ru/γAl2O3 in a CREC-Riser Simulator" Catalysts 12, no. 3: 282. https://doi.org/10.3390/catal12030282