Enhanced Photoredox Activity of BiVO4/Prussian Blue Nanocomposites for Efficient Pollutant Removal from Aqueous Media under Low-Cost LEDs Illumination
Abstract
:1. Introduction
2. Results and Discussion
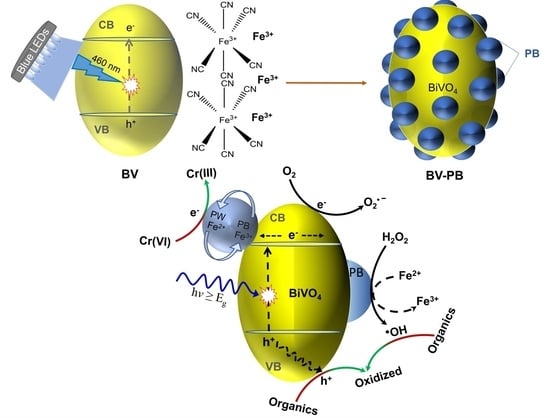
2.1. Modification of BV with PB
2.2. Morphological Analysis of BV and BV-PB
2.3. Structural and Phase Analysis by XRD
2.4. Vibrational Spectroscopic (Raman and FTIR) Analysis
2.5. Optical Properties
2.6. Photocatalytic Properties
2.6.1. Photooxidation of MB dye
2.6.2. Photoreduction of Cr(VI)
2.7. Why BV-PB Shows Enhanced Photoactivity?
- 1.
- Enhanced absorption of visible light (Figure 7),
- 2.
- The ability of Fe centers in PB to produce reactive oxygen species (ROS) in a Fenton-like derived process,
- 3.
- The role of PB as cocatalysts, lowering resistance to charge transfer and improving charge transfer ability at the photocatalysts/solution interface, thus promoting electron transfer from the conduction band (CB) of BV to the Cr(VI) and/or O2 species in solution, and/or
- 4.
- Decreased electron-hole recombination due to lower charge transfer resistance.
3. Materials and Methods
3.1. Reagents
3.2. Preparation of BV and BV-PB Photocatalysts
3.3. Characterization Techniques
3.4. Evaluation of Photocatalytic Activity
3.4.1. Photocatalytic Reduction of Cr(VI)
3.4.2. Photocatalytic Degradation of Methylene Blue (MB)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saiz, P.G.; Valverde, A.; Gonzalez-Navarrete, B.; Rosales, M.; Quintero, Y.M.; Fidalgo-Marijuan, A.; Orive, J.; Reizabal, A.; Larrea, E.S.; Arriortua, M.I.; et al. Modulation of the Bifunctional CrVI to CrIII Photoreduction and Adsorption Capacity in ZrIV and TiIV Benchmark Metal-Organic Frameworks. Catalysts 2021, 11, 51. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of Hexavalent Chromium on the Environment and Removal Techniques: A Review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Y.; Xu, X.; Xiang, Y.; Yang, Z.; Wang, P. Highly Efficient Photocatalytic Cr(VI) Reduction by Lead Molybdate Wrapped with D-A Conjugated Polymer under Visible Light. Catalysts 2021, 11, 106. [Google Scholar] [CrossRef]
- Szabó, M.; Kalmár, J.; Ditrói, T.; Bellér, G.; Lente, G.; Simic, N.; Fábián, I. Equilibria and Kinetics of Chromium(VI) Speciation in Aqueous Solution—A Comprehensive Study from PH 2 to 11. Inorganica Chim. Acta 2018, 472, 295–301. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and Genotoxic Effects of Hexavalent Chromium in Environment and Its Bioremediation Strategies. J. Environ. Sci. Health Part C 2016, 34, 1–32. [Google Scholar] [CrossRef]
- WHO Expert Consultation for 2nd Addendum to the 3rd Edition of the Guidelines for Drinking-Water Quality: Geneva, 15–19 May 2006, WHO/SDE/WSH/06.05 v, 136 p. Available online: https://Apps.Who.Int/Iris/Handle/10665/69604 (accessed on 28 July 2022).
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.V.; Bhatnagar, A. Modified Biochar as a Green Adsorbent for Removal of Hexavalent Chromium from Various Environmental Matrices: Mechanisms, Methods, and Prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Eltaweil, A.S. Novel Reusable Amine-Functionalized Cellulose Acetate Beads Impregnated Aminated Graphene Oxide for Adsorptive Removal of Hexavalent Chromium Ions. Int. J. Biol. Macromol. 2022, 208, 925–934. [Google Scholar] [CrossRef]
- Du, J.; Shang, X.; Shi, J.; Guan, Y. Removal of Chromium from Industrial Wastewater by Magnetic Flocculation Treatment: Experimental Studies and PSO-BP Modelling. J. Water Process Eng. 2022, 47, 102822. [Google Scholar] [CrossRef]
- Salman, R.H.; Hassan, H.A.; Abed, K.M.; Al-Alawy, A.F.; Tuama, D.A.; Hussein, K.M.; Jabir, H.A. Removal of Chromium Ions from a Real Wastewater of Leather Industry Using Electrocoagulation and Reverse Osmosis Processes. AIP Conf. Proc. 2020, 2213, 020186. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Dong, X.; Meng, Q.W.; Ge, Q. Positively Charged Membranes Constructed via Complexation for Chromium Removal through Micellar-Enhanced Forward Osmosis. Chem. Eng. J. 2021, 420, 129837. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Chen, Q. Electrochemical Treatment Of Landfill Leachate To Remove Chromium (Vi) Using Ni3n And Nio Nps Anodes. Int. J. Electrochem. Sci. 2021, 16, 210710. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Zhou, X.; Li, Z.; Zhao, C.; Jia, X.; Ji, M. Bio-Immobilization and Recovery of Chromium Using a Denitrifying Biofilm System: Identification of Reaction Zone, Binding Forms and End Products. J. Environ. Sci. 2023, 126, 70–80. [Google Scholar] [CrossRef]
- Rathna, T.; PonnanEttiyappan, J.B.; RubenSudhakar, D. Fabrication of Visible-Light Assisted TiO2-WO3-PANI Membrane for Effective Reduction of Chromium (VI) in Photocatalytic Membrane Reactor. Environ. Technol. Innov. 2021, 24, 102023. [Google Scholar] [CrossRef]
- Haji Ali, B.; Baghdadi, M.; Torabian, A. Application of Nickel Foam as an Effective Electrode for the Electrochemical Treatment of Liquid Hazardous Wastes of COD Analysis Containing Mercury, Silver, and Chromium (VI). Environ. Technol. Innov. 2021, 23, 101617. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Thakur, A.K.; Gupta, A.; Arnusch, C.J. Electrochemical Removal of Organic and Inorganic Pollutants Using Robust Laser-Induced Graphene Membranes. ACS Appl. Mater. Interfaces 2021, 13, 1452–1462. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Perissinotto, A.P.; de Vicente, F.S.; Ribeiro, S.J.L.; Worsley, M.A.; Rodrigues-Filho, U.P. Prussian Blue as a Co-Catalyst for Enhanced Cr(vi) Photocatalytic Reduction Promoted by Titania-Based Nanoparticles and Aerogels. New J. Chem. 2021, 45, 10217–10231. [Google Scholar] [CrossRef]
- Yu, M.; Shang, J.; Kuang, Y. Efficient Photocatalytic Reduction of Chromium (VI) Using Photoreduced Graphene Oxide as Photocatalyst under Visible Light Irradiation. J. Mater. Sci Technol. 2021, 91, 17–27. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Serge-correales, Y.E.; Ullah, S.; Ferreira-Neto, E.P.; Rojas-mantilla, H.D.; Hazra, C.; Ribeiro, S.J.L. A UV-Visible-NIR Active Smart Photocatalytic System Based on NaYbF4:Tm3+ Upconverting Particles and Ag3PO4/H2O2 for Photocatalytic Processes under Light on/Light off Conditions. Mater. Adv. 2022, 3, 2706–2715. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Khan, M.Y.; Khan, A.; Harunsani, M.H. Visible Light Active La-Doped Ag3PO4 for Photocatalytic Degradation of Dyes and Reduction of Cr(VI). Solid State Sci. 2022, 131, 106950. [Google Scholar] [CrossRef]
- Ullah, S.; Fayeza; Khan, A.A.; Jan, A.; Aain, S.Q.; Neto, E.P.F.; Serge-Correales, Y.E.; Parveen, R.; Wender, H.; Rodrigues-Filho, U.P.; et al. Enhanced Photoactivity of BiVO4/Ag/Ag2O Z-Scheme Photocatalyst for Efficient Environmental Remediation under Natural Sunlight and Low-Cost LED Illumination. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124946. [Google Scholar] [CrossRef]
- Gomes, L.E.; Nogueira, A.C.; da Silva, M.F.; Plaça, L.F.; Maia, L.J.Q.; Gonçalves, R.V.; Ullah, S.; Khan, S.; Wender, H. Enhanced Photocatalytic Activity of BiVO4/Pt/PtOx Photocatalyst: The Role of Pt Oxidation State. Appl. Surf. Sci. 2021, 567, 150773. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.P.; Hazra, C.; Parveen, R.; Rojas-Mantilla, H.D.D.; Calegaro, M.L.L.; Serge-Correales, Y.E.E.; Rodrigues-Filho, U.P.P.; Ribeiro, S.J.L.J.L. Broad Spectrum Photocatalytic System Based on BiVO4 and NaYbF4:Tm3+ Upconversion Particles for Environmental Remediation under UV-Vis-NIR Illumination. Appl. Catal. B 2019, 243, 121–135. [Google Scholar] [CrossRef]
- Shahzad, K.; Tahir, M.B.; Sagir, M.; Kabli, M.R. Role of CuCo2S4 in Z-Scheme MoSe2/BiVO4 Composite for Efficient Photocatalytic Reduction of Heavy Metals. Ceram. Int. 2019, 45, 23225–23232. [Google Scholar] [CrossRef]
- Yin, G.; Liu, C.; Shi, T.; Ji, D.; Yao, Y.; Chen, Z. Porous BiVO4 Coupled with CuFeO2 and NiFe Layered Double Hydroxide as Highly-Efficient Photoanode toward Boosted Photoelectrochemical Water Oxidation. J. Photochem. Photobiol. A Chem. 2022, 426, 113742. [Google Scholar] [CrossRef]
- Li, S.; Xing, Z.; Feng, J.; Yan, L.; Wei, D.; Wang, H.; Wu, D.; Ma, H.; Fan, D.; Wei, Q. A Sensitive Biosensor of CdS Sensitized BiVO4/GaON Composite for the Photoelectrochemical Immunoassay of Procalcitonin. Sens. Actuators B Chem. 2021, 329, 129244. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Wu, S.H.; Wang, Q.M.; Sun, J.J. A Novel Split-Type Photoelectrochemical Biosensor Based on Double-Strand Specific Nuclease-Assisted Cycle Amplification Coupled with Plasmonic Ag Enhanced BiVO4 Performance for Sensitive Detection of MicroRNA-155. Sens. Actuators B Chem. 2022, 369, 132251. [Google Scholar] [CrossRef]
- Ho-Kimura, S.; Soontornchaiyakul, W.; Yamaguchi, Y.; Kudo, A. Preparation of Nanoparticle Porous-Structured BiVO4 Photoanodes by a New Two-Step Electrochemical Deposition Method for Water Splitting. Catalysts 2021, 11, 136. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Huang, X.; Wang, Y.; Xu, Y. Improved Performance of BiVO4 via Surface-Deposited Magnetic CuFe2O4 for Phenol Oxidation and O2 Reduction and Evolution under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 45776–45784. [Google Scholar] [CrossRef]
- Alhaddad, M.; Amin, M.S.; Zaki, Z.I. Novel BiVO4/ZnO Heterojunction for Amended Photoreduction of Mercury (II) Ions. Opt. Mater. 2022, 127, 112251. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, S.; He, J.; Long, F.; Wang, L. In Situ Synthesis of BiVO4/BiOBr Microsphere Heterojunction with Enhanced Photocatalytic Performance. J. Alloys Compd. 2022, 927, 166661. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Cui, X.; Zhu, J.; Li, Y.; Zhang, P.; Li, J. Direct Z-Scheme Nanoporous BiVO4/CdS Quantum Dots Heterojunction Composites as Photoanodes for Photocathodic Protection of 316 Stainless Steel under Visible Light. Appl. Surf. Sci. 2022, 603, 154394. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Gao, L.; Yang, D.; Wen, S.; Huang, W.; Sun, Z.; Guo, J.; Jiang, X.; Lu, C. Fabrication of Ternary Dual Z-Scheme AgI/ZnIn2S4/BiVO4 Heterojunction Photocatalyst with Enhanced Photocatalytic Degradation of Tetracycline under Visible Light. Arab. J. Chem. 2022, 15, 104159. [Google Scholar] [CrossRef]
- Pai, H.; Kuo, T.R.; Chung, R.J.; Kubendhiran, S.; Yougbaré, S.; Lin, L.Y. Enhanced Photocurrent Density for Photoelectrochemical Catalyzing Water Oxidation Using Novel W-Doped BiVO4 and Metal Organic Framework Composites. J. Colloid Interface Sci. 2022, 624, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Maheskumar, V.; Jiang, Z.; Lin, Y.; Vidhya, B. The Structural and Optical Properties of Ag/Cu Co-Doped BiVO4 Material: A Density Functional Study. Mater. Lett. 2022, 315, 131289. [Google Scholar] [CrossRef]
- Prabhavathy, S.; Arivuoli, D. Visible Light-Induced Silver and Lanthanum Co-Doped BiVO4 Nanoparticles for Photocatalytic Dye Degradation of Organic Pollutants. Inorg. Chem. Commun. 2022, 141, 109483. [Google Scholar] [CrossRef]
- Sun, W.; Dong, Y.; Zhai, X.; Zhang, M.; Li, K.; Wang, Q.; Ding, Y. Crystal Facet Engineering of BiVO4/CQDs/TPP with Improved Charge Transfer Efficiency for Photocatalytic Water Oxidation. Chem. Eng. J. 2022, 430, 132872. [Google Scholar] [CrossRef]
- Srinivasan, N.; Anbuchezhiyan, M.; Harish, S.; Ponnusamy, S. Efficient Catalytic Activity of BiVO4 Nanostructures by Crystal Facet Regulation for Environmental Remediation. Chemosphere 2022, 289, 133097. [Google Scholar] [CrossRef]
- Kadam, A.N.; Babu, B.; Lee, S.-W.; Kim, J.; Yoo, K. Morphological Guided Sphere to Dendrite BiVO4 for Highly Efficient Organic Pollutant Removal and Photoelectrochemical Performance under Solar Light. Chemosphere 2022, 305, 135461. [Google Scholar] [CrossRef]
- Maheskumar, V.; Lin, Y.M.; Jiang, Z.; Vidhya, B.; Ghosal, A. New Insights into the Structural, Optical, Electronic and Photocatalytic Properties of Sulfur Doped Bulk BiVO4 and Surface BiVO4 on {0 1 0} and {1 1 0} via a Collective Theoretical and Experimental Investigation. J. Photochem. Photobiol. A Chem. 2022, 426, 113757. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.R.; Khiadani, M.; Zhong, Y.; Wang, W.; Su, C.; Shao, Z. Prussian Blue-Conjugated ZnO Nanoparticles for near-Infrared Light-Responsive Photocatalysis. Mater. Today Energy 2022, 23, 100895. [Google Scholar] [CrossRef]
- Tada, H.; Tsuji, S.; Ito, S. Photodeposition of Prussian Blue Films on TiO2: Additive Effect of Methanol and Influence of the TiO2 Crystal Form. J. Colloid Interface Sci. 2001, 239, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, C.; Qian, M.; Xiao, P.; Ge, P.; Shen, C.; Wu, X.L.; Chen, J. Hollow-Structured Amorphous Prussian Blue Decorated on Graphitic Carbon Nitride for Photo-Assisted Activation of Peroxymonosulfate. J. Colloid Interface Sci. 2021, 603, 856–863. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kim, W.; Lee, W.; Kim, S. Photocatalytic Enhancement of Cesium Removal by Prussian Blue-Deposited TiO2. J. Hazard. Mater. 2018, 357, 449–456. [Google Scholar] [CrossRef]
- Song, Y.Y.; Zhang, K.; Xia, X.H. Photosynthesis and Characterization of Prussian Blue Nanocubes on Surfaces of TiO2 Colloids. Appl. Phys. Lett. 2006, 88, 053112. [Google Scholar] [CrossRef]
- Maurin-Pasturel, G.; Long, J.; Guari, Y.; Godiard, F.; Willinger, M.G.; Guerin, C.; Larionova, J. Nanosized Heterostructures of Au@Prussian Blue Analogues: Towards Multifunctionality at the Nanoscale. Angew. Chem. Int. Ed. 2014, 53, 3872–3876. [Google Scholar] [CrossRef]
- Uchida, H.; Sasaki, T.; Ogura, K. Dark Catalytic Reduction of CO2 over Prussian Blue-Deposited TiO2 and the Photo-Reactivation of the Catalyst. J. Mol. Catal. 1994, 93, 269–277. [Google Scholar] [CrossRef]
- Ghobadi, T.G.U.; Ghobadi, A.; Soydan, M.C.; Vishlaghi, M.B.; Kaya, S.; Karadas, F.; Ozbay, E. Strong Light–Matter Interactions in Au Plasmonic Nanoantennas Coupled with Prussian Blue Catalyst on BiVO4 for Photoelectrochemical Water Splitting. ChemSusChem 2020, 13, 2577–2588. [Google Scholar] [CrossRef]
- Meng, X.; Xu, S.; Zhang, C.; Feng, P.; Li, R.; Guan, H.; Ding, Y. Prussian Blue Type Cocatalysts for Enhancing the Photocatalytic Water Oxidation Performance of BiVO4. Chem. A Eur. J. 2022, 28, e202201407. [Google Scholar] [CrossRef]
- Li, J.; Chu, Y.; Zhang, C.; Zhang, X.; Wu, C.; Xiong, X.; Zhou, L.; Wu, C.; Han, D. CoFe Prussian Blue Decorated BiVO4 as Novel Photoanode for Continuous Photocathodic Protection of 304 Stainless Steel. J. Alloy. Compd. 2021, 887, 161279. [Google Scholar] [CrossRef]
- Yuan, D.; Sun, M.; Tang, S.; Zhang, Y.; Wang, Z.; Qi, J.; Rao, Y.; Zhang, Q. All-Solid-State BiVO4/ZnIn2S4 Z-Scheme Composite with Efficient Charge Separations for Improved Visible Light Photocatalytic Organics Degradation. Chin. Chem. Lett. 2020, 31, 547–550. [Google Scholar] [CrossRef]
- Yuan, D.; Sun, M.; Zhao, M.; Tang, S.; Qi, J.; Zhang, X.; Wang, K.; Li, B. Persulfate Promoted ZnIn2S4 Visible Light Photocatalytic Dye Decomposition. Int. J. Electrochem. Sci. 2020, 15, 8761–8770. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C.H. LED-Based Photocatalytic Treatment of Pesticides and Chlorophenols. J. Environ. Eng. 2014, 139, 1146–1151. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C. Application of Photocatalysts and LED Light Sources in Drinking Water Treatment. Catalysts 2013, 3, 726–743. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Saito, Y.; Kawahara, H. Photodeposition of Prussian Blue on TiO2 Particles. J. Electrochem. Soc. 1991, 138, 140–144. [Google Scholar] [CrossRef]
- Jang, S.C.; Hong, S.B.; Yang, H.M.; Lee, K.W.; Moon, J.K.; Seo, B.K.; Huh, Y.S.; Roh, C. Removal of Radioactive Cesium Using Prussian Blue Magnetic Nanoparticles. Nanomaterials 2014, 4, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Baggio, B.F.; Vicente, C.; Pelegrini, S.; Cid, C.C.P.; Brandt, I.S.; Tumelero, M.A.; Pasa, A.A. Morphology and Structure of Electrodeposited Prussian Blue and Prussian White Thin Films. Materials 2019, 12, 1103. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Packiaraj, R.; Venkatesh, K.S.; Devendran, P.; Bahadur, S.A.; Nallamuthu, N. Structural, Morphological and Electrochemical Studies of Nanostructured BiVO4 for Supercapacitor Application. Mater. Sci. Semicond. Process. 2020, 115, 105122. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M. Facile Hydrothermal Synthesis of Egg-like BiVO4 Nanostructures for Photocatalytic Desulfurization of Thiophene under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 17735–17740. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Malik, M.A.; Denca, A.; Strojek, J. In Situ FT-IR/ATR Spectroelectrochemistry of Prussian Blue in the Solid State. Anal. Chem. 1996, 68, 2442–2446. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, J.; Zhao, X.; Liu, H. Fabrication and Photoelectrocatalytic Properties of Nanocrystalline Monoclinic BiVO4 Thin-Film Electrode. J. Environ. Sci. 2011, 23, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Rykov, A.I.; Sharma, V.K.; Wei, H.; Jin, C.; Liu, X.; Li, M.; Yu, S.; Sun, C.; et al. Prussian Blue/TiO2 Nanocomposites as a Heterogeneous Photo-Fenton Catalyst for Degradation of Organic Pollutants in Water Xuning. Catal. Sci. Technol. 2015, 5, 504–514. [Google Scholar] [CrossRef]
- Cheng, K.L.; Ueno, K.; Imamura, T. Handbook of Organic Analytical Reagents, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992; ISBN 9781315140568. [Google Scholar]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of Active Oxidative Species in TiO2 Photocatalysis Using the Fluorescence Technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; Marchiori, L.; Ferreira-Neto, E.P.; Wender, H.; Parveen, R.; Muneeb, M.; Mattos, B.O.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L.; Ullah, S. Enhanced Photoredox Activity of BiVO4/Prussian Blue Nanocomposites for Efficient Pollutant Removal from Aqueous Media under Low-Cost LEDs Illumination. Catalysts 2022, 12, 1612. https://doi.org/10.3390/catal12121612
Khan AA, Marchiori L, Ferreira-Neto EP, Wender H, Parveen R, Muneeb M, Mattos BO, Rodrigues-Filho UP, Ribeiro SJL, Ullah S. Enhanced Photoredox Activity of BiVO4/Prussian Blue Nanocomposites for Efficient Pollutant Removal from Aqueous Media under Low-Cost LEDs Illumination. Catalysts. 2022; 12(12):1612. https://doi.org/10.3390/catal12121612
Chicago/Turabian StyleKhan, Abrar Ali, Leonardo Marchiori, Elias Paiva Ferreira-Neto, Heberton Wender, Rashida Parveen, Mohammad Muneeb, Bianca Oliveira Mattos, Ubirajara Pereira Rodrigues-Filho, Sidney José Lima Ribeiro, and Sajjad Ullah. 2022. "Enhanced Photoredox Activity of BiVO4/Prussian Blue Nanocomposites for Efficient Pollutant Removal from Aqueous Media under Low-Cost LEDs Illumination" Catalysts 12, no. 12: 1612. https://doi.org/10.3390/catal12121612