Photocatalytic Concrete Developed by Short Seedless Hydrothermal Method for Water Purification
Abstract
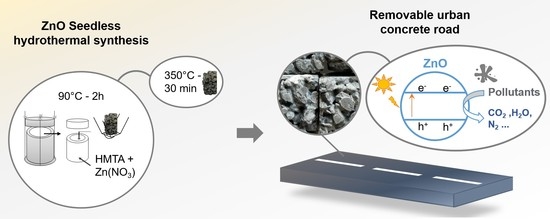
:1. Introduction
2. Results and Discussion
2.1. Concrete Surface Characterization
2.1.1. Concrete Surface Observations and SEM Analysis
- The turbulence and obstruction caused by the concrete surface complex microstructure, leading to the formation of aggregates and fusion of the ZnO NSs;
- The concrete basic pH property and the chemical composition with different adjuvants used, which could act as structure-modifying agents during the hydrothermal growth [5]. Hydroxyl ions (OH−) excess and structure-modifying agent, such as trisodium citrate (Na3C6H5O7), were indeed assigned to the NSHs, NSHSs, and HAs formation by being selectively adsorbed on the positively charged zinc (001) plane, leading to a total or partial suppression of growth along the (002) direction, thus favoring the growth in other directions [5,15,16,17].
2.1.2. Gap Measurement
2.1.3. XRD Characterization
2.2. Photocatalytic Activity Evaluation
2.2.1. Methyl Orange Removal
- The fine texture of NSs and the presence of numerous boundaries increasing dye adsorption and light harvesting;
- The hierarchical structure, the various gains and interfaces facilitating the electron/hole (e−/h+) photogeneration, and the diffusion of e/h;
- The rich mesoporous structure helping dye adsorption and light-generated charge transfer.
2.2.2. Acid Red 14 Removal
3. Materials and Methods
3.1. Concrete Surface Functionalization
3.1.1. Two-Step Hydrothermal Synthesis Method
3.1.2. One-Step Hydrothermal Synthesis Method
3.2. Photocatalytic Activity Evaluation: Water Purification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, X.; Cui, S.; Li, H.; Abdelhady, A.; Wang, H.; Zhou, H. Removal effect on stormwater runoff pollution of porous concrete treated with nanometer titanium dioxide. Trans. Res. Part D 2019, 73, 34–45. [Google Scholar] [CrossRef]
- Shen, S.; Burton, M.; Jobson, B.; Haselbach, L. Pervious concrete with titanium dioxide as photocatalyst compound for a greener urban road environment. Const. Build. Mater. 2012, 35, 874–883. [Google Scholar] [CrossRef]
- Asadi, S.; Hassan, A.M.; Kevern, J.T.; Rupnow, T.D. Development of photocatalytic pervious concrete pavement for air and storm water improvements. J. Trans. Res. Board 2012, 2290, 161–167. [Google Scholar] [CrossRef]
- Le Pivert, M.; Poupart, R.; Capochichi-Gnambodoe, M.; Martin, N.; Leprince-Wang, Y. Direct growth of ZnO nanowires on civil engineering materials: Smart materials for supported photodegradation. Micro. Nanoengin. 2019, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Le Pivert, M.; Zerelli, B.; Martin, N.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Smart ZnO decorated optimized engineering materials for water purification under natural sunlight. Const. Build. Mater. 2020, 257, 119592. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, L.; Xu, L.; Shen, Y.; Wang, L.; Liu, Y. Shaped-controlled growth of sphere-like ZnO on modified polyester fabric in water bath. Mater. Lett. 2021, 288, 129342. [Google Scholar] [CrossRef]
- Pastor, A.; Balbuena, J.; Cruz-Yusta, B.M.; Pavlovic, I.; Schànchez, L. ZnO on rice husk: A sustainable photocatalyst for urban air purification. Chem. Eng. J. 2019, 368, 659–667. [Google Scholar] [CrossRef]
- Choudhary, S.; Sahu, K.; Bisht, A.; Satpati, B.; Mohapatra, S. Rapid synthesis of ZnO nanowires and nanoplates with highly enhanced photocatalytic performance. App. Surf. Sci. 2021, 541, 148484. [Google Scholar] [CrossRef]
- Chandran, R.; Mallik, A. Facile, seedless and sufractant-free synthesis of ZnO nanostructures by wet chemical bath method and their characterization. Appl. Nanosci. 2018, 8, 1823–1830. [Google Scholar] [CrossRef]
- Le Pivert, M.; Kerivel, O.; Zerelli, B.; Leprince-Wang, Y. ZnO nanostructures based innovative photocatalytic road for air purification. J. Clean. Prod. 2021, 318, 128447. [Google Scholar] [CrossRef]
- Hossain, M.F.; Naka, S.; Okada, H. Fabrication of perovskite solar cells with ZnO nanostructures prepared on seedless ITO substrate. J. Mater. Sci. Mater. Elec. 2018, 29, 13864–13871. [Google Scholar] [CrossRef]
- Hong, G.W.; Kim, J.; Lee, J.S.; Shin, K.; Jung, D.; Kim, J.H. A flexible tactile sensor using seedless hydrothermal growth of ZnO nanorods on fabrics. J. Phys. Com. 2020, 4, 045002. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Yeom, J. A flotable piezo-photocatalytic platform based on semi-embedded ZnO nanowire array for high-performance water decontamination. Nano-Micro Lett. 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Serairi, L.; Leprince-Wang, Y. Enhanced photocatalytic activity of ZnO nanostructure for water purification. Phys. Status Solidi B 2016, 253, 1480–1484. [Google Scholar] [CrossRef]
- Shaban, M.; Zayed, M.; Hamdy, H. Nanostructured ZnO thin films for self-cleaning applications. R. Soc. Chem. 2017, 7, 617–631. [Google Scholar] [CrossRef]
- Khoa, N.T.; Kim, S.W.; Thuan, D.V.; Yoo, D.H.; Kim, E.J.; Hahn, S.H. Hydrothermally controlled ZnO nanosheet self-assembled hollow spheres/hierarchical aggregates and their photocatalytic activities. CrystEngComm 2013, 16, 1344–1350. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Xiang, L.; Wang, J. Hydrothermal synthesis of (001) facet highly exposed ZnO plates: A new insight into the effect of citrate. Crystals 2019, 9, 552. [Google Scholar] [CrossRef] [Green Version]
- Sedran, T.; Gennesseaux, E.; Nguyen, M.-L.; Waligora, J.; Guyot, D.; Le Mouel, J. Development of a permeable removable urban pavement. In Proceedings of the International Conference on Concrete Roads, Krakow, Poland, 25–28 June 2023. [Google Scholar]
- Wen, X.; Wu, W.; Ding, Y.; Wang, L.Z. Seedless synthesis of patterned ZnO nanowire arrays on metal thin films (Au, Ag, Cu, Sn) and their application for flexible electromechanical sensing. J. Mater. Chem. 2012, 22, 9469–9476. [Google Scholar] [CrossRef]
- Mai, H.H.; Tran, D.H.; Janssens, E. Non-enzymatic fluorescent glucose sensor using vertically aligned ZnO nanotubes grown by a one-step, seedless hydrothermal method. Microchim. Acta 2019, 186, 245. [Google Scholar] [CrossRef]
- Tian, J.H.; Hu, J.; Li, S.S.; Zhang, F.; Liu, J.; Shi, J.; Li, X.; Tian, Z.Q.; Chen, Y. Improved seedless hydrothermal synthesis of dense and ultralong ZnO nanowires. Nanotechnology 2011, 22, 245601. [Google Scholar] [CrossRef]
- Adbulrahman, A.F.; Ahmed, S.M.; Hamad, S.M.; Almessiere, M.A.; Sadaji, S.M. Effect of different pH values on growth solutions for the ZnO nanostructures. J. Phys. 2021, 71, 175–189. [Google Scholar] [CrossRef]
- Bilal, H.; Chen, T.; Ren, M.; Gao, X.; Su, A. Influence of silica fume, metakaolin & SBR latex on strength and durability performance of previous concrete. Cons. Build. Mater. 2021, 275, 122124. [Google Scholar] [CrossRef]
- Winkler, N.; Edinger, S.; Kautek, W.; Dimopoulos, T. Mg-doped ZnO films prepared by chemical bath deposition. J. Mater. Sci. 2018, 53, 5159–5171. [Google Scholar] [CrossRef] [Green Version]
- Leprince-Wang, Y.; Martin, N.; Ghozlane Habba, Y.; Le Pivert, M.; Capochichi-Gnambodoe, M. ZnO nanostructure based photocatalysis for water purification. Nanoworld J. 2020, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Samadi, M.; Shivaee, H.A.; Pourjavadi, A.; Moshfegh, A.Z. Synergism of oxygen vacancy and carbonaceous species on enhanced photocatalytic activity of electrospun ZnO-carbon nanofibers: Charge carrier scavengers mechanism. Appl. Catal. A Gen. 2013, 466, 153–160. [Google Scholar] [CrossRef]
- Yu, Z.; Moussa, H.; Liu, M.; Schneider, R.; Moliere, M.; Liao, H. Solution precursor plasma spray process as an alternative rapid one-step route for the development of hierarchical ZnO films for improved photocatalytic degradation. Ceram. Int. 2018, 44, 2085–2092. [Google Scholar] [CrossRef]
- Yu, Z.; Moussa, H.; Chouchene, R.; Schneider, R.; Wang, W.; Moliere, M.; Liao, H. Tunable morphologies of ZnO films via the solution precursor plasma spray process for improved photocatalytic degradation performance. Appl. Surf. Sci. 2018, 455, 970–979. [Google Scholar] [CrossRef]
- Joshi, S.; Jones, L.A.; Sabri, Y.M.; Bhargava, S.K.; Sunkara, M.V.; Ippolito, S.J. Facile conversion of zinc hydroxide carbonate to CaO-ZnO for selective CO2 detection. J. Colloid Interface Sci. 2020, 588, 310–322. [Google Scholar] [CrossRef]
- Dash, P.; Manna, A.; Mishra, N.C.; Varma, S. Synthesis and characterization of aligned ZnO nanorods for visible light photocatalysis. Phys. E Low-Dimens. Sys. Nanostructures 2019, 107, 38–46. [Google Scholar] [CrossRef]
- Haghighat Mamaghani, A.; Haghighat, F.; Lee, C.S. Effect of titanium dioxide properties and support material on photocatalytic oxidation of indoor air pollutants. Build. Environ. 2021, 189, 107518. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M. Bulk phase degradation of acid red 14 by nanophotocatalysis using immobilize titanium(IV) oxide nanoparticles. J. Photochem. Photobiol. A Chem. 2006, 182, 60–66. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Heidari-Asil, S.A.; Salavati-Niasari, M. Recyclable magnetic ZnCo2O4-based ceramics nanostructure materials fabricated by simple sonochemical route for effective sunlight-driven photocatalytic degradation of organic pollution. Ceram. Int. 2021, 47, 8959–8979. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Pivert, M.; Leprince-Wang, Y. Photocatalytic Concrete Developed by Short Seedless Hydrothermal Method for Water Purification. Catalysts 2022, 12, 1620. https://doi.org/10.3390/catal12121620
Le Pivert M, Leprince-Wang Y. Photocatalytic Concrete Developed by Short Seedless Hydrothermal Method for Water Purification. Catalysts. 2022; 12(12):1620. https://doi.org/10.3390/catal12121620
Chicago/Turabian StyleLe Pivert, Marie, and Yamin Leprince-Wang. 2022. "Photocatalytic Concrete Developed by Short Seedless Hydrothermal Method for Water Purification" Catalysts 12, no. 12: 1620. https://doi.org/10.3390/catal12121620