Enhanced Photocatalytic Degradation of P-Chlorophenol by ZnIn2S4 Nanoflowers Modified with Carbon Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and Performance Valuation
2.2. Degradation Products and Pathways
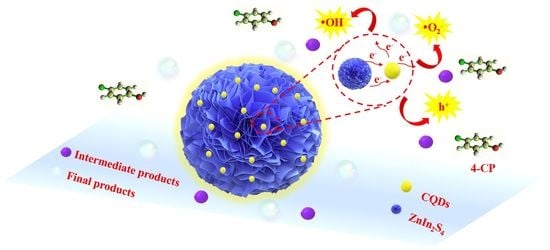
2.3. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemical Reagents
3.2. Characterizations
3.3. Materials Preparation
3.3.1. Synthesis of ZnIn2S4
3.3.2. Synthesis of CQDs
3.3.3. Synthesis of CQDs/ZnIn2S4-x
3.4. Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, K.C.; Zhao, Y.X.; Ji, M.; Li, Z.L.; Zhai, S.Y.; Zhou, X.; Wang, Q.; Wang, C.; Liang, B. Challenges and opportunities for the biodegradation of chlorophenols: Aerobic, anaerobic and bioelectrochemical processes. Water Res. 2021, 193, 116862. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Wang, J.L. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Wang, M.L.; Fang, G.D.; Liu, P.; Zhou, D.M.; Ma, C.; Zhang, D.J.; Zhan, J.H. Fe3O4@beta-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl. Catal. B 2016, 188, 113–122. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Barrera-Rendon, E.; Oliva, J.; Rodriguez-Gonzalez, V. Flexible CuS/TiO2 based composites made with recycled bags and polystyrene for the efficient removal of the 4-CP pesticide from drinking water. Sep. Purif. Technol. 2021, 270, 118821. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.B.; Ma, Q.L.; Li, D.F.; Wang, X.M.; Gao, W.S.; Wang, H.; Wang, X.L.; Li, Z.L.; Li, C. Highly Efficient Degradation of Persistent Pollutants with 3D Nanocone TiO2-Based Photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [CrossRef]
- Peng, Y.Q.; Chen, J.H.; Lu, S.Y.; Huang, J.X.; Zhang, M.M.; Buekens, A.; Li, X.D.; Yan, J.H. Chlorophenols in Municipal Solid Waste Incineration: A review. Chem. Eng. J. 2016, 292, 398–414. [Google Scholar] [CrossRef]
- Liu, J.; Han, D.D.; Chen, P.J.; Zhai, L.P.; Wang, Y.J.; Chen, W.H.; Mi, L.W.; Yang, L.P. Positive roles of Br in g-C3N4/PTCDI-Br heterojunction for photocatalytic degrading chlorophenols. Chem. Eng. J. 2021, 418, 129492. [Google Scholar] [CrossRef]
- Gong, Q.J.; Liu, Y.; Dang, Z. Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2,4-dichlorophenol degradation under visible light. J. Hazard. Mater. 2019, 371, 677–686. [Google Scholar] [CrossRef]
- Ding, M.; Zhou, J.J.; Yang, H.C.; Cao, R.Y.; Zhang, S.W.; Shao, M.H.; Xu, X.J. Synthesis of Z-scheme g-C3N4 nanosheets/Ag3PO4 photocatalysts with enhanced visible-light photocatalytic performance for the degradation of tetracycline and dye. Chin. Chem. Lett. 2020, 31, 71–76. [Google Scholar] [CrossRef]
- Hu, D.H.; Song, L.J.; Yan, R.; Li, Z.J.; Zhang, Z.Q.; Sun, J.H.; Bian, J.; Qu, Y.; Jing, L.Q. Valence-mixed iron phthalocyanines/(100) Bi2MoO6 nanosheet Z-scheme heterojunction catalysts for efficient visible-light degradation of 2-chloro-phenol via preferential dechlorination. Chem. Eng. J. 2022, 440, 135786. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. ZnIn2S4 and ZnIn2S4 based advanced hybrid materials: Structure, morphology and applications in environment and energy. Inorg. Chem. Commun. 2022, 138, 109288. [Google Scholar] [CrossRef]
- Yuan, D.L.; Sun, M.T.; Tang, S.F.; Zhang, Y.T.; Wang, Z.T.; Qi, J.B.; Rao, Y.D.; Zhang, Q.R. All-solid-state BiVO4/ZnIn2S4 Z-scheme composite with efficient charge separations for improved visible light photocatalytic organics degradation. Chin. Chem. Lett. 2020, 31, 547–550. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, H.; Shi, Z.W.; Wu, F.L.; Guo, J.; Deng, K.M.; Li, L. 2D ZnIn2S4 Nanosheet/lD TiO2 Nanorod Heterostructure Arrays for Improved Photoelectrochemical Water Splitting. ACS Appl. Mater. Inter. 2014, 6, 17200–17207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Lin, Z.Y.; Yang, G.W. A 2D self-assembled MoS2/ZnIn2S4 heterostructure for efficient photocatalytic hydrogen evolution. Nanoscale 2017, 9, 18290–18298. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Liu, Y.H.; Mao, B.D.; Tan, L.L.; Yang, Y.L.; Shi, W.D. Ag doping of Zn-In-S quantum dots for photocatalytic hydrogen evolution: Simultaneous bandgap narrowing and carrier lifetime elongation. Appl. Catal. B 2017, 216, 11–19. [Google Scholar] [CrossRef]
- Zuo, G.C.; Wang, Y.T.; Teo, W.L.; Xian, Q.M.; Zhao, Y.L. Direct Z-scheme TiO2-ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B 2021, 291, 120126. [Google Scholar] [CrossRef]
- Lai, J.H.; Jiang, X.Y.; Zhao, M.; Cui, S.A.; Yang, J.; Li, Y.F. Thickness-dependent layered BiOIO3 modified with carbon quantum dots for photodegradation of bisphenol A: Mechanism, pathways and DFT calculation. Appl. Catal. B 2021, 298, 120622. [Google Scholar] [CrossRef]
- Ren, P.; Fu, X.B.; Zhang, Y.M. Carbon Quantum Dots-TiO2 Nanocomposites with Enhanced Catalytic Activities for Selective Liquid Phase Oxidation of Alcohols. Catal. Lett. 2017, 147, 1679–1685. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, X.; Meng, X.; Chen, T.X.; Kong, W.Y.; Li, Y.; Zhao, Y.X.; Wang, D.W.; Zhu, S.M.; Cheema, W.A.; et al. Enhanced visible/near-infrared light harvesting and superior charge separation via 0D/2D all-carbon hybrid architecture for photocatalytic oxygen evolution. Carbon 2020, 167, 724–735. [Google Scholar] [CrossRef]
- Fu, Y.K.; Zeng, G.M.; Lai, C.; Huang, D.L.; Qin, L.; Yi, H.; Liu, X.G.; Zhang, M.M.; Li, B.S.; Liu, S.Y. Hybrid architectures based on noble metals and carbon-based dots nanomaterials: A review of recent progress in synthesis and applications. Chem. Eng. J. 2020, 399, 125743. [Google Scholar] [CrossRef]
- Yu, H.B.; Huang, J.H.; Jiang, L.B.; Shi, Y.H.; Yi, K.X.; Zhang, W.; Zhang, J.; Chen, H.Y.; Yuan, X.Z. Enhanced photocatalytic tetracycline degradation using N-CQDs/OV-BiOBr composites. Chem. Eng. J. 2020, 402, 126187. [Google Scholar] [CrossRef]
- Jiang, C.L.; Wang, H.; Wang, Y.Q.; Ji, H.B. All solid-state Z-scheme CeO2/ZnIn2S4 hybrid for the photocatalytic selective oxidation of aromatic alcohols coupled with hydrogen evolution. Appl. Catal. B 2020, 277, 119235. [Google Scholar] [CrossRef]
- Shao, Y.Q.; Dou, Z.L.; Liang, X.Y.; Zhang, X.X.; Ji, M.; Pang, M.; Wang, M.; Wang, X.K. ZnIn2S4 nanosheet growth on amine-functionalized SiO2 for the photocatalytic reduction of CO2. Catal. Sci. Technol. 2022, 12, 606–612. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Zeng, H.H.; Lin, H.; Li, H.X.; Huang, L.L.; Tham, K.O.; Mohamed, A.R.; Lim, J.W. Enhanced synchronous photocatalytic 4-chlorophenol degradation and Cr (VI) reduction by novel magnetic separable visible-light-driven Z-scheme CoFe2O4/P-doped BiOBr heterojunction nanocomposites. Environ. Res. 2022, 212, 113394. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Chen, T.; Yang, N.; Wang, S.Y.; Ding, X.; Chen, H. Deep insight into ROS mediated direct and hydroxylated dichlorination process for efficient photocatalytic sodium pentachlorophenate mineralization. Appl. Catal. B 2021, 296, 120352. [Google Scholar] [CrossRef]
- Xiang, X.F.; Wu, L.Y.; Zhu, J.J.; Li, J.Z.; Liao, X.; Huang, H.C.; Fan, J.J.; Lv, K.L. Photocatalytic degradation of sulfadiazine in suspensions of TiO2 nanosheets with exposed (001) facets. Chin. Chem. Lett. 2021, 32, 3215–3220. [Google Scholar] [CrossRef]
- Huang, S.H.; Wang, Y.; Wan, J.Q.; Yan, Z.C.; Ma, Y.W.; Zhang, G.H.; Wang, S.L. Ti3C2Tx as electron-hole transfer mediators to enhance AgBr/BiOBr Z heterojunction photocatalytic for the degradation of Tetrabromobisphenol A: Mechanism Insight. Appl. Catal. B 2022, 319, 121913. [Google Scholar] [CrossRef]
- Qin, C.D.; Tang, J.J.; Qiao, R.X.; Lin, S.J. Tetracycline sensitizes TiO2 for visible light photocatalytic degradation via ligand-to-metal charge transfer. Chin. Chem. Lett. 2022, 33, 5218–5222. [Google Scholar] [CrossRef]
- Xu, X.J.; Tang, D.D.; Cai, J.H.; Xi, B.D.; Zhang, Y.; Pi, L.; Mao, X.H. Heterogeneous activation of peroxymonocarbonate by chalcopyrite (CuFeS2) for efficient degradation of 2,4-dichlorophenol in simulated groundwater. Appl. Catal. B 2019, 251, 273–282. [Google Scholar] [CrossRef]
- Huang, S.N.; Tian, F.; Dai, J.W.; Tian, X.S.; Li, G.F.; Liu, Y.L.; Chen, Z.Q.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and O-1(2) involved process. Appl. Catal. B 2021, 298, 120576. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Y.; Yang, S.R.; Lin, J.Y.; Lu, J.H.; Song, W.; Zhu, S.J.; Wang, Z.H. Selective degradation of parachlorophenol using Fe/Fe3O4@CPPy nanocomposites via the dual nonradical/radical peroxymonosulfate activation mechanisms. Chem. Eng. J. 2022, 445, 136806. [Google Scholar] [CrossRef]
- Ge, T.T.; Han, J.Y.; Qi, Y.M.; Gu, X.Y.; Ma, L.; Zhang, C.; Naeem, S.; Huang, D.J. The toxic effects of chlorophenols and associated mechanisms in fish. Aquat. Toxicol. 2017, 184, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, J.; Chi, H.Z.; Ding, A.; Xin, Y.J.; Ma, Y.Y.; Liu, Q.L.; He, X. Novel VUV/g-C3N4 system with high adaptability to varied environmental conditions and outstanding degradation capacity for chlorophenols. J. Hazard. Mater. 2021, 419, 126473. [Google Scholar] [CrossRef]
- Wang, A.W.; Ni, J.X.; Wang, W.; Liu, D.M.; Zhu, Q.; Xue, B.X.; Chang, C.C.; Ma, J.; Zhao, Y. MOF Derived Co-Fe nitrogen doped graphite carbon@crosslinked magnetic chitosan Micro-nanoreactor for environmental applications: Synergy enhancement effect of adsorption-PMS activation. Appl. Catal. B 2022, 319, 121926. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Hollow SnO2 nanotubes decorated with ZnIn2S4 nanosheets for enhanced visible-light photocatalytic activity. J. Alloy Compd. 2020, 843, 155772. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [PubMed]
- Liu, B.B.; Liu, X.J.; Li, L.; Li, J.W.; Li, C.; Gong, Y.Y.; Niu, L.Y.; Zhao, X.S.; Sun, C.Q. ZnIn2S4 flowerlike microspheres embedded with carbon quantum dots for efficient photocatalytic reduction of Cr(VI). Chin. J. Catal. 2018, 39, 1901–1909. [Google Scholar] [CrossRef]
- Xu, H.Q.; Jiang, Y.H.; Yang, X.Y.; Li, F.; Li, A.P.; Liu, Y.; Zhang, J.M.; Zhou, Z.Z.; Ni, L. Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation. Mater. Res. Bull. 2018, 97, 158–168. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Yin, S.; Xu, H.; Xu, L.i.; Xu, Y.; He, M.; Li, H. Preparation of sphere-like g-C3N4/BiOI photocatalysts via a reactable ionic liquid for visible-light-driven photocatalytic degradation of pollutants. J. Mater. Chem. A 2014, 2, 5340. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.i.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defectsmediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Ismael, M.; Elhaddad, E.; Taffa, D.H.; Wark, M. Solid state route for synthesis of YFeO3/g-C3N4 composites and its visible light activity for degradation of organic pollutants. Catal. Today 2018, 313, 47–54. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Liu, Q.; Qiu, Y.; Liu, F.; Wang, F. Enhanced Photocatalytic Degradation of P-Chlorophenol by ZnIn2S4 Nanoflowers Modified with Carbon Quantum Dots. Catalysts 2022, 12, 1545. https://doi.org/10.3390/catal12121545
Qiu J, Liu Q, Qiu Y, Liu F, Wang F. Enhanced Photocatalytic Degradation of P-Chlorophenol by ZnIn2S4 Nanoflowers Modified with Carbon Quantum Dots. Catalysts. 2022; 12(12):1545. https://doi.org/10.3390/catal12121545
Chicago/Turabian StyleQiu, Jinli, Quan Liu, Yixing Qiu, Fuqiang Liu, and Fenghe Wang. 2022. "Enhanced Photocatalytic Degradation of P-Chlorophenol by ZnIn2S4 Nanoflowers Modified with Carbon Quantum Dots" Catalysts 12, no. 12: 1545. https://doi.org/10.3390/catal12121545