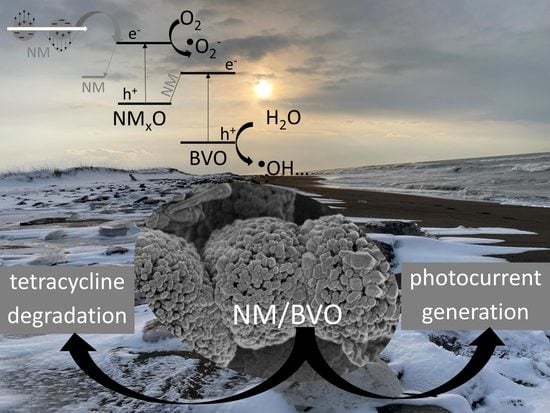
Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and Analysis
2.2. Photocatalytic Activities of Samples
2.3. Mechanism of the Photocatalytic Degradation of Organic Compounds on NM-Modified BVO
3. Experiment
3.1. Preparation of BVO and NM/BVO
3.2. Characterization
3.3. Photocatalytic and Electrochemical Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Kholuiskaya, S.N.; Dillert, R.; Kulak, A.I.; Kokorin, A.I. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Appl. Catal. B Environ. 2002, 36, 161–169. [Google Scholar] [CrossRef]
- Esswein, A.J.; Nocera, D.G. Hydrogen production by molecular photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhou, Y.; Wang, J.; Tang, J.; Wang, L.; Feng, C. Facile fabrication of mediator-free Z-scheme photocatalyst of phosphorous doped ultrathin graphitic carbon nitride nanosheets and bismuth vanadate composites with enhanced tetracycline degradation under visible light. J. Colloid Interface Sci. 2018, 509, 219–234. [Google Scholar] [CrossRef]
- Cheng, L.; Jiang, T.; Yan, K.; Gong, J.; Zhang, J. A dual-cathode photoelectrocatalysis-electroenzymatic catalysis system by coupling BiVO4 photoanode with hemin/Cu and carbon cloth cathodes for degradation of tetracycline. Electrochim. Acta 2019, 298, 561–569. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photoch. Photobio. C 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Role of titanium dioxide (TiO2) structural design/morphology in photocatalytic air purification. Appl. Catal. B Environ. 2020, 269, 118735. [Google Scholar] [CrossRef]
- Huang, D.; Wen, M.; Zhou, C.; Li, Z.; Cheng, M.; Chen, S.; Xue, W.; Lei, L.; Yang, Y.; Xiong, W.; et al. ZnxCd1-XS based materials for photocatalytic hydrogen evolution, pollutants degradation and carbon dioxide reduction. Appl. Catal. B Environ. 2020, 267, 118651. [Google Scholar] [CrossRef]
- Skoutelis, C.; Antonopoulou, M.; Konstantinou, I.; Vlastos, D.; Papadaki, M. Photodegradation of 2-chloropyridine in aqueous solution: Reaction pathways and genotoxicity of intermediate products. J. Hazard. Mater. 2017, 321, 753–763. [Google Scholar] [CrossRef]
- Kudo, A. Recent progress in the development of visible light-driven powdered photocatalysts for water splitting. Int. J. Hydrogen Energy 2007, 32, 2673–2678. [Google Scholar] [CrossRef]
- Tong, R.; Sun, Z.; Zhong, X.; Wang, X.; Xu, J.; Yang, Y.; Xu, B.; Wang, S.; Pan, H. Enhancement of visible-light photocatalytic hydrogen production by CeCO3OH in G-C3N4/CeO2 System. ChemCatChem 2019, 11, 1069–1075. [Google Scholar] [CrossRef]
- Hussain, T.; Junaid, M.; Qayyum, H.A. Preparation of Ba-doped SrTiO3 photocatalyst by sol-gel method for hydrogen generation. Chem. Phys. Lett. 2020, 754, 137741. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Gregor, M.; Satrapinskyy, L.; Raptis, D.; Lianos, P.; Plesch, G. Photooxidative properties of various BiVO4/TiO2 layered composite films and study of their photocatalytic mechanism in pollutant degradation. J. Environ. Chem. Eng. 2017, 5, 5143–5149. [Google Scholar] [CrossRef]
- Nair, A.K.; Roy George, D.; Jos Baby, N.; Reji, M.; Joseph, S. Solar dye degradation using TiO2 nanosheet based nanocomposite floating photocatalyst. Mater. Today Proc. 2021, 46, 2747–2751. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Caceres, J.; Fernandez-Alba, A.R.; Aguera, A.; Rodriguez, A. Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catal. Today 2002, 76, 209–220. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Arzaee, N.A.; Mohamad Noh, M.F.; Ismail, A.F.; Safaei, J.; Sagu, J.S.; Johan, M.R.; Mat Teridi, M.A. Electrodeposition of BiVO4 with needle-like flower architecture for high performance photoelectrochemical splitting of water. Ceram. Int. 2021, 47, 24227–24239. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.-K. The effects of W/Mo-Co-doped BiVO4 photoanodes for improving photoelectrochemical water splitting performance. Catal. Today 2021, 361, 183–190. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Jayabalan, P.J.; Ong, W.-J.; Ng, Y.H.; Sufian, S. Photocatalytic degradation of real industrial poultry wastewater via platinum decorated BiVO4/g-C3N4 photocatalyst under solar light irradiation. J. Photochem. Photobiol. A 2019, 378, 46–56. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Fu, F.; Li, W. Effects of PH on the hierarchical structures and photocatalytic performance of Cu-doped BiVO4 prepared via the hydrothermal method. Mater. Sci. Semicond. Process. 2015, 35, 197–206. [Google Scholar] [CrossRef]
- Yao, M.; Liu, M.; Gan, L.; Zhao, F.; Fan, X.; Zhu, D.; Xu, Z.; Hao, Z.; Chen, L. Monoclinic mesoporous BiVO4: Synthesis and visible-light-driven photocatalytic property. Colloids Surf. A 2013, 433, 132–138. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. On the different photocatalytic performance of BiVO4 catalysts for methylene blue and rhodamine B degradation. J. Mol. Catal. A Chem. 2013, 376, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Zhang, H.; Xu, H.; Zhang, Y. M-BiVO4 hollow spheres coated on carbon fiber with superior reusability as photocatalyst. Colloids Surf. A 2017, 531, 213–220. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Xu, P.; Gong, X.; Xue, W.; Lei, L.; Deng, R.; Li, J.; Li, Z. Facet-engineered surface and interface design of monoclinic scheelite bismuth vanadate for enhanced photocatalytic performance. ACS Catal. 2020, 10, 1024–1059. [Google Scholar] [CrossRef]
- Lamdab, U.; Wetchakun, K.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. InVO4–BiVO4 composite films with enhanced visible light performance for photodegradation of methylene blue. Catal. Today 2016, 278, 291–302. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Wang, Y.; Cao, D.; Tian, S.; Xiao, K.; Mao, R.; Zhao, X. 2-H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation. Chem. Eng. J. 2018, 332, 312–320. [Google Scholar] [CrossRef]
- Ramadan, W.; Dillert, R.; Koch, J.; Tegenkamp, C.; Bahnemann, D.W. Changes in the solid-state properties of bismuth iron oxide during the photocatalytic reformation of formic acid. Catal. Today 2019, 326, 22–29. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.-J.; Zhang, J.-X.; Bai, F.-Q.; Zhang, H.-X. First-principles investigation on the interfacial interaction and electronic structure of BiVO4/WO3 heterostructure semiconductor material. Appl. Surf. Sci. 2021, 549, 149309. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zhang, M.; Liu, S.; Yi, H.; Liu, X.; An, N.; Zhou, X.; Li, L.; Fu, Y.; et al. N, S-GQDs and Au nanoparticles Co-modified ultrathin Bi2MoO6 nanosheet with enhanced charge transport dynamics for full-spectrum-light-driven molecular oxygen activation. Chem. Eng. J. 2021, 409, 128281. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Deng, J.; Liu, Y.; Au, C.T. Effect of sulfur doping on the photocatalytic performance of BiVO4 under visible light illumination. Chin. J. Catal. 2013, 34, 1617–1626. [Google Scholar] [CrossRef]
- Khazaee, Z.; Khavar, A.H.C.; Mahjoub, A.R.; Motaee, A.; Srivastava, V.; Sillanpää, M. Template-confined growth of X-Bi2MoO6 (X: F, Cl, Br, I) nanoplates with open surfaces for photocatalytic oxidation: Experimental and DFT insights of the halogen doping. Sol. Energy 2020, 196, 567–581. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Huang, G.; Fu, S.; Ma, C.; Wang, B.; Huang, Q.; Liao, H. Hydrothermal synthesis and enhanced photocatalytic activity of hierarchical flower-like Fe-doped BiVO4. Trans. Nonferrous Met. Soc. China 2017, 27, 868–875. [Google Scholar] [CrossRef]
- Cai, J.; Xia, Y.; Gang, R.; He, S.; Komarneni, S. Activation of MoS2 via tungsten doping for efficient photocatalytic oxidation of gaseous mercury. Appl. Catal. B Environ. 2022, 314, 121486. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, N.; Luo, M.; Fan, L.; Zhao, K.; Qu, J.; Guan, J.; Yuan, X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 Type II Heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. [Google Scholar] [CrossRef]
- Kumar, R.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Saini, A.; Saini, V.; Singh, P. An overview on bismuth molybdate based photocatalytic systems: Controlled morphology and enhancement strategies for photocatalytic water purification. J. Environ. Chem. Eng. 2020, 8, 104291. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.-W.; Swihart, M.T.; Yoon, S.S. Morphology engineering of photoelectrodes for efficient photoelectrochemical water splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Lei, N.; Song, Q.; Liang, Z. Morphological evolution and enhanced photoelectrochemical performance of V4+ self-doped, [010] oriented BiVO4 for water splitting. J. Alloys Compd. 2019, 771, 914–923. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Wang, Q.; Jiang, X.; Shen, Y. Enhanced photoelectrochemical water splitting using a Cobalt-sulfide-decorated BiVO4 photoanode. Chin. J. Catal. 2022, 43, 433–441. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak, E.; Choina, J.; Ziebro, J.; Morawski, A.W. Enhanced adsorption of two azo dyes produced by carbon modification of TiO2. Desalination 2009, 249, 359–363. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Wang, F.; Liu, X.; Ren, C.; Miao, X. Fabrication of Pt nanoparticles decorated Gd-doped Bi2MoO6 nanosheets: Design, radicals regulating and mechanism of Gd/Pt-Bi2MoO6 photocatalyst. Appl. Surf. Sci. 2018, 427, 1046–1053. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, M.; Ma, D.; Jing, D. Effect of preparation parameters on photoactivity of BiVO4 by hydrothermal method. J. Nanomater. 2012, 2012, 1–6. [Google Scholar]
- Phiankoh, S.; Munprom, R. Effect of PH on crystal structure and morphology of hydrothermally-synthesized BiVO4. Mater. Today Proc. 2018, 5, 9447–9452. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Courbon, H.; Pichat, P. Regioselective isotopic exchange between propane and deuterium over illuminated PtTiO2 catalyst below room temperature. J. Catal. 1987, 108, 426–432. [Google Scholar] [CrossRef]
- Fan, H.; Wang, D.; Xie, T.; Lin, Y. The preparation of high photocatalytic activity nano-spindly Ag-BiVO4 and photoinduced carriers transfer properties. Chem. Phys. Lett. 2015, 640, 188–193. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shiota, S.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Au nanoparticles supported on BiVO4: Effective inorganic photocatalysts for H2O2 production from water and O2 under visible light. ACS Catal. 2016, 6, 4976–4982. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, K.; Li, S.; Wang, X.; Wang, P.; Chen, F.; Yu, H. Mass-Transfer Control for Selective Deposition of Well-Dispersed AuPd Cocatalysts to Boost Photocatalytic H2O2 Production of BiVO4. Chem. Eng. J. 2022, 443, 136429. [Google Scholar] [CrossRef]
- Li, S.; Xue, B.; Chen, J.; Liu, Y.; Zhang, J.; Wang, H.; Liu, J. Constructing a plasmonic p-n heterojunction photocatalyst of 3D Ag/Ag6Si2O7/Bi2MoO6 for efficiently removing broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 254, 117579. [Google Scholar] [CrossRef]
- Bi, J.; Fang, W.; Li, L.; Li, X.; Liu, M.; Liang, S.; Zhang, Z.; He, Y.; Lin, H.; Wu, L.; et al. Ternary reduced-graphene-oxide/Bi2MoO6/Au nanocomposites with enhanced photocatalytic activity under visible light. J. Alloys Compd. 2015, 649, 28–34. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Zhou, Z.; Wang, P.; Mi, X.; Xia, Y.; Wang, H.; Zhan, S.; Li, Y.; Li, L. Plasmonic ag as electron-transfer mediators in Bi2MoO6/Ag-AgCl for efficient photocatalytic inactivation of bacteria. Chem. Eng. J. 2020, 382, 122762. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, C.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic enhancement in BiVO4 photonic crystals for efficient water splitting. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Yi, G.; Zhang, Z.; Zhang, X.; Li, P.; Zhang, C.; Chen, L.; Zhang, Y.; Sun, Q. Fabrication of Ag particles deposited BiVO4 photoanode for significantly efficient visible-light driven photoelectrocatalytic degradation of β-Naphthol. J. Environ. Chem. Eng. 2022, 10, 107221. [Google Scholar] [CrossRef]
- Li, H.; Hong, W.; Cui, Y.; Hu, X.; Fan, S.; Zhu, L. Enhancement of the visible light photocatalytic activity of Cu2O/BiVO4 catalysts synthesized by ultrasonic dispersion method at room temperature. Mater. Sci. Eng. B 2014, 181, 1–8. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Zeng, H.; Zhang, J.; Wang, Y.; Sillanpää, M.; Zhao, X. Enhanced photoelectrocatalytic degradation of bisphenol a by BiVO4 photoanode coupling with peroxymonosulfate. J. Hazard. Mater. 2020, 394, 121105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef] [PubMed]












| Samples | Content (at.%) | Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bi | V | O | C | Me | V/Bi | O/Bi | C/Bi | Me/Bi | |
| BVO | 11.6 | 17.4 | 38.6 | 32.2 | - | 1.5 | 3.3 | 2.8 | - |
| Au/BVO | 11.0 | 12.4 | 38.2 | 39.9 | 1.0 | 1.1 | 3.5 | 3.6 | 9.1 |
| Ag/BVO | 10.2 | 11.3 | 39.7 | 38.4 | 1.1 | 1.1 | 3.9 | 3.8 | 10.9 |
| Cu/BVO | 7.4 | 15.9 | 48.3 | 33.2 | 1.0 | 2.2 | 6.5 | 4.5 | 13.5 |
| Pt/BVO | 7.1 | 6.3 | 39.6 | 45.3 | 0.7 | 0.9 | 5.6 | 6.4 | 9.9 |
| Pd/BVO | 10.4 | 14.0 | 39.4 | 35.3 | 0.1 | 1.4 | 3.8 | 3.4 | 0.9 |
| Element | Au | Ag | Cu | ||||
| Form | Au(δ+) | Au(0) | Au(δ−) | Ag(+) | Ag(0) | Cu(2+) | Cu(+) |
| content | 6.5% | 55.8% | 37.7% | 93.0% | 7.0% | 17.2% | 82.8% |
| Element | Pd | Pt | |||||
| Form | Pd(4+) | Pd(2+) | Pd(0) | Pt(2+) | Pt(0) | ||
| content | 11.9% | 29% | 59.1% | 15.9% | 84.1% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Yue, X.; Chang, Y.; Wang, K.; Zhang, J.; Sun, J.; Wei, Z.; Kowalska, E. Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals. Catalysts 2022, 12, 1293. https://doi.org/10.3390/catal12111293
Wu L, Yue X, Chang Y, Wang K, Zhang J, Sun J, Wei Z, Kowalska E. Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals. Catalysts. 2022; 12(11):1293. https://doi.org/10.3390/catal12111293
Chicago/Turabian StyleWu, Limeng, Xin Yue, Ying Chang, Kunlei Wang, Jinyue Zhang, Jiajie Sun, Zhishun Wei, and Ewa Kowalska. 2022. "Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals" Catalysts 12, no. 11: 1293. https://doi.org/10.3390/catal12111293