Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon
Abstract
:1. Introduction
2. Results
2.1. Characterization of a Carbon Support
2.2. Characterization of the Active Phase (Cobalt) Particles
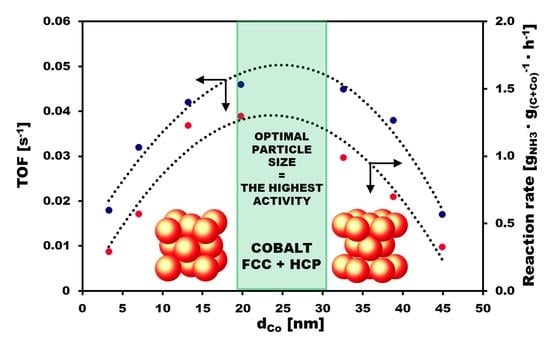
2.3. Catalyst Activity
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Activity Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boudart, M. Catalysis by Supported Metals. Adv. Catal. 1969, 20, 153–166. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Carrazza, J. Structure sensitivity of catalytic reactions. J. Ind. Eng. Chem. Fundam. 1986, 25, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Samorjai, G.A. The structure sensitivity and insensitivity of catalytic reactions in light of the adsorbate induced dynamic restructuring of surfaces. Catal. Lett. 1990, 7, 169–182. [Google Scholar] [CrossRef]
- Van Santen, R.A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 2009, 42, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Strongin, D.R.; Carrazza, J.; Bare, S.R.; Somorjai, G.A. The importance of C7 sites and surface roughness in the ammonia synthesis reaction over iron. J. Catal. 1987, 103, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.J.H.; Dahl, S.; Hansen, P.L.; Törnqvist, E.; Jensen, L.; Topsøe, H.; Prip, D.V.; Møenshaug, P.B.; Chorkendorff, I. Structure sensitivity of supported ruthenium catalysts for ammonia synthesis. J. Mol. Catal. A Chem. 2000, 163, 19–26. [Google Scholar] [CrossRef]
- Kitakami, O.; Sato, H.; Shimada, Y.; Sato, F.; Tanaka, M. Size effect on the crystal phase of cobalt fine particles. Phys. Rev. B 1997, 56, 13849–13854. [Google Scholar] [CrossRef]
- Bozso, F.; Ertl, G.; Grunze, M.; Weiss, M. Interaction of nitrogen with iron surfaces: I. Fe(100) and Fe(111). J. Catal. 1977, 49, 18–41. [Google Scholar] [CrossRef]
- Bozso, F.; Ertl, G.; Weiss, M. Interaction of nitrogen with iron surfaces: II. Fe(110). J. Catal. 1977, 50, 519–529. [Google Scholar] [CrossRef]
- Spencer, N.D.; Schoonmaker, R.C.; Somorjai, G.A. Iron single crystals as ammonia synthesis catalysts: Effect of surface structure on catalyst activity. J. Catal. 1982, 74, 129–135. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Topsøe, H.; Khammouma, S.; Boudart, M. Surface, catalytic and magnetic properties of small iron particles: II. Structure sensitivity of ammonia synthesis. J. Catal. 1975, 37, 503–512. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Topsøe, H.; Boudart, M. Surface, catalytic and magnetic properties of small iron particles: III. Nitrogen induced surface reconstruction. J. Catal. 1975, 37, 513–522. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Materer, N. Surface structures in ammonia synthesis. Top. Catal. 1994, 1, 215–231. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Su, H.-Y.; Liu, J.-X.; Li, W.-X. Interplay Between Site Activity and Density of BCC Iron for Ammonia Synthesis Based on First-Principles Theory. ChemCatChem 2019, 11, 1928–1934. [Google Scholar] [CrossRef]
- Parker, I.B.; Waugh, K.C.; Bowker, M. On the structure sensitivity of ammonia synthesis on promoted and unpromoted iron. J. Catal. 1988, 114, 457–459. [Google Scholar] [CrossRef]
- Qian, J.; An, Q.; Fortunelli, A.; Nielsen, R.J.; Goddard, W.A., III. Reaction Mechanism and Kinetics for Ammonia Synthesis on the Fe(111) Surface. J. Am. Chem. Soc. 2018, 140, 6288–6297. [Google Scholar] [CrossRef] [Green Version]
- Dahl, S.; Logadottir, A.; Egeberg, R.C.; Larsen, J.H.; Chorkendorff, I.; Törnqvist, E.; Nørskov, J.K. Role of Steps in N2 Activation on Ru(0001). Phys. Rev. Lett. 1999, 83, 1814–1817. [Google Scholar] [CrossRef] [Green Version]
- Dahl, S.; Törnqvist, E.; Chorkendorff, I. Dissociative adsorption of N2 on Ru(0001): A surface reaction totally dominated by steps. J. Catal. 2000, 192, 381–390. [Google Scholar] [CrossRef]
- Dahl, S.; Sehested, J.; Jacobsen, C.J.H.; Törnqvist, E.; Chorkendorff, I. Surface science based microkinetic analysis of ammonia synthesis over ruthenium catalysts. J. Catal. 2000, 192, 391–399. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Van Montfoort, A. The influence of crystallite size on the adsorption of molecular nitrogen on nickel, palladium and platinum: An infrared and electron-microscopic study. Surf. Sci. 1966, 4, 396–430. [Google Scholar] [CrossRef]
- Liang, C.; Wei, Z.; Xin, Q.; Li, C. Ammonia synthesis over Ru/C catalysts with different carbon supports promoted by barium and potassium compounds. Appl. Catal. A Gen. 2001, 208, 193–201. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Szmigiel, D.; Kowalczyk, Z. Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitized carbon. J. Catal. 2005, 231, 11–19. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.-F.; Zhang, T.; Cai, H.; Zhou, Y.; Lin, B.; Lin, X.; Zheng, Y.; Zheng, L.; Wang, X.; et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: From nanoparticles to subnanometric clusters and atomic clusters. Chem 2022, 8, 749–768. [Google Scholar] [CrossRef]
- Rambeau, G.; Jorti, A.; Amariglio, H. Catalytic activity of a cobalt powder in NH3 synthesis in relation with the allotropic transformation of the metal. J. Catal. 1985, 94, 155–165. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Chen, P.-P.; Liu, J.-X.; Su, H.-Y.; Li, W.-X. Influence of Cobalt Crystal Structures on Activation of Nitrogen Molecule: A First-Principles Study. J. Phys. Chem. C 2019, 123, 10956–10966. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Kępiński, L.; Kaszkur, Z.; Kielar, K.; Kowalczyk, Z. Ammonia synthesis over barium-promoted cobalt catalysts supported on graphitized carbon. J. Catal. 2007, 249, 24–33. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Matyszek, M.; Kaszkur, Z.; Kępiński, L.; Kowalczyk, Z. Carbon-supported cobalt catalyst for ammonia synthesis: Effect of preparation procedure. J. Catal. 2006, 237, 207–210. [Google Scholar] [CrossRef]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.; Teunissen, H.T.; Chorkendorff, I. Ammonia synthesis with barium-promoted iron–cobalt alloys supported on carbon. J. Catal. 2003, 214, 327–335. [Google Scholar] [CrossRef]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.; Teunissen, H.T.; Stahl, K.; Chorkendorff, I. New efficient catalyst for ammonia synthesis: Barium-promoted cobalt on carbon. Chem. Commun. 2002, 11, 1206–1207. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Karolewska, M.; Truszkiewicz, E.; Iwanek, E.; Mierzwa, B. Cobalt catalyst doped with cerium and barium obtained by Co-precipitation method for ammonia synthesis process. Catal. Lett. 2011, 141, 678–684. [Google Scholar] [CrossRef]
- Tarka, A.; Patkowski, W.; Zybert, M.; Ronduda, H.; Wieciński, P.; Adamski, P.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis. Catalysts 2020, 10, 658. [Google Scholar] [CrossRef]
- Cao, A.; Bukas, V.J.; Shadravan, V.; Wang, Z.; Li, H.; Kibsgaard, J.; Chorkendorff, I.; Norskøv, J.K. A spin promotion effect in catalytic ammonia synthesis. Nat. Commun. 2022, 13, 2382. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Sentek, J.; Jodzis, S.; Diduszko, R.; Presz, A.; Terzyk, A.; Kucharski, Z.; Suwalski, J. Thermally modified active carbon as a support for catalysts for NH3 synthesis. Carbon 1996, 34, 403–409. [Google Scholar] [CrossRef]
- Toth, P. Nanostructure quantification of turbostratic carbon by HRTEM image analysis: State of the art, biases, sensitivity and best practices. Carbon 2021, 178, 688–707. [Google Scholar] [CrossRef]
- Ruz, P.; Banerjee, S.; Pandey, M.; Sudarsan, V.; Sastry, P.U.; Kshirsagar, R.J. Structural evolution of turbostratic carbon: Implications in H2 storage. Solid State Sci. 2016, 62, 105–111. [Google Scholar] [CrossRef]
- Zhong, Z.; Aika, K. The Effect of Hydrogen Treatment of Active Carbon on Ru Catalysts for Ammonia Synthesis. J. Catal. 1998, 173, 535–539. [Google Scholar] [CrossRef]
- Zhong, Z.; Aika, K. Effect of ruthenium precursor on hydrogen-treated active carbon supported ruthenium catalysts for ammonia synthesis. Inorg. Chem. Acta 1998, 280, 183–188. [Google Scholar] [CrossRef]
- Aika, K.; Hori, H.; Ozaki, A. Activation of nitrogen by alkali metal promoted transition metal I. Ammonia synthesis over ruthenium promoted by alkali metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Szmigiel, D.; Komornicki, A.; Zieliński, J.; Kowalczyk, Z. Catalytic properties of small ruthenium particles deposited on carbon: Ammonia decomposition studies. Carbon 2003, 41, 589–591. [Google Scholar] [CrossRef]
- Bonarowska, M.; Raróg-Pilecka, W.; Karpiński, Z. The use of active carbon pretreated at 2173 K as a support for palladium catalysts for hydrodechlorination reactions. Catal. Today 2011, 169, 223–231. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Reuel, R.C.; Bartholomew, C.H. The stoichiometries of H2 and CO adsorptions on cobalt: Effects of support and preparation. J. Catal. 1984, 85, 63–77. [Google Scholar] [CrossRef]
- Borodziński, A.; Bonarowska, M. Relation between Crystallite Size and Dispersion on Supported Metal Catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]
- Kowalczyk, Z. Effect of potassium on the high pressure kinetics of ammonia synthesis over fused iron catalyst. Catal. Lett. 1996, 37, 173–179. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Tarka, A.; Ostrowski, A.; Raróg-Pilecka, W. Kinetic studies of ammonia synthesis over a barium-promoted cobalt catalyst supported on magnesium–lanthanum mixed oxide. J. Taiwan Inst. Chem. Eng. 2020, 114, 241–248. [Google Scholar] [CrossRef]






| Catalyst | dCo-fcc 1 (nm) | dcore-shell 2 (nm) | dcore 3 (nm) |
|---|---|---|---|
| Ba-Co4.9/C | 14 | – | - |
| Ba-Co9.5/C | 21 | 20 | 15 |
| Ba-Co28.2/C | 19 | 26 | 19 |
| Ba-Co43.1/C | 18 | 22 | 16 |
| Ba-Co67.7/C | 25 | 41 | 31 |
| Catalyst | H2 Uptake (μmol gCo−1) | FE (%) 1 | dCo (nm) 2 |
|---|---|---|---|
| Ba-Co4.9/C | 2740 | 32.5 | 3 |
| Ba-Co9.5/C | 1530 | 18.0 | 7 |
| Ba-Co28.2/C | 850 | 9.5 | 13 |
| Ba-Co43.1/C | 540 | 6.3 | 20 |
| Ba-Co52.9/C | 330 | 3.9 | 33 |
| Ba-Co59.4/C | 270 | 3.2 | 39 |
| Ba-Co67.7/C | 240 | 2.8 | 45 |
| Catalyst | rNH3 (gNH3 g(C+Co) −1 h−1) | Reference |
|---|---|---|
| Ba-Co28.2/C | 1.23 | This work |
| Ba-Co43.1/C | 1.30 | This work |
| Ba-Co22.8N/B | 0.86 | [26] |
| Ba-Co25.4N(R/P+C)/B | 1.05 | [26] |
| Ba-CoR+P+C/C | 0.65 | [27] |
| KM I | 0.33 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zybert, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon. Catalysts 2022, 12, 1285. https://doi.org/10.3390/catal12101285
Zybert M, Tarka A, Patkowski W, Ronduda H, Mierzwa B, Kępiński L, Raróg-Pilecka W. Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon. Catalysts. 2022; 12(10):1285. https://doi.org/10.3390/catal12101285
Chicago/Turabian StyleZybert, Magdalena, Aleksandra Tarka, Wojciech Patkowski, Hubert Ronduda, Bogusław Mierzwa, Leszek Kępiński, and Wioletta Raróg-Pilecka. 2022. "Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon" Catalysts 12, no. 10: 1285. https://doi.org/10.3390/catal12101285