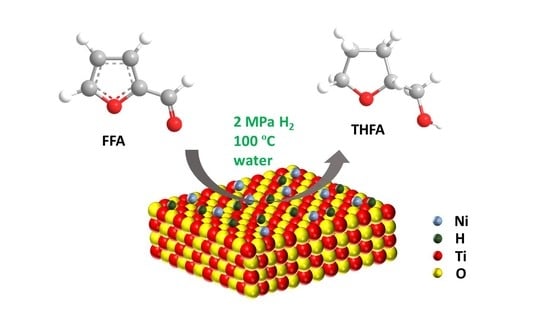
Spillover Hydrogen on Electron-Rich Ni/m-TiO2 for Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol
Abstract
:1. Introduction
2. Results & Discussion
2.1. Characterization Results
2.2. Catalytic Activity
3. Material and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohre, A.; Dutta, S.; Saha, B.; Abu-omar, M.M. Upgrading Furfurals to Drop-in Biofuels: An Overview. ACS Sustain. Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- Ahorsu, R.; Constanti, M.; Medina, F. Recent Impacts of Heterogeneous Catalysis in Biorefineries. Ind. Eng. Chem. Res. 2021, 60, 18612–18626. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Saravanamurugan, S.; Yang, S. Catalytic Upgrading of Biomass-Derived Sugars with Acidic Nanoporous Materials: Structural Role in Carbon-Chain Length Variation. ChemSusChem 2019, 12, 347–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.T.L.; Wu, T.Y. A Review on Solvent Systems for Furfural Production from Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2021, 137, 110172. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, P.; Li, Q.; Xia, H. Recent Advances in the Catalytic Conversion of Biomass to Furfural in Deep Eutectic Solvents. Front. Chem. 2022, 10, 911674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent Advances in the Conversion of Furfural into Bio-Chemicals through Chemo-And Bio-Catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.; Salagre, P.; González, M.D.; Llorca, J.; Cesteros, Y. Effect of the Formation of NiCu Alloy and Use of Biomass-Derived Furfural on the Catalytic Hydrogenation of Furfural to THFA. Mol. Catal. 2020, 490, 110956. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Fu, P.; Liu, X.; Xie, M.; Jiang, S.; Wen, H.; Zhou, Y.; Wang, J. Palladium Confined in Pure-Silica TON Zeolite for Furfuryl Alcohol Hydrogenation into Tetrahydrofurfuryl Alcohol. Microporous Mesoporous Mater. 2021, 322, 111161. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Lan, X.; Wang, T. Supported Ultrafine NiCo Bimetallic Alloy Nanoparticles Derived from Bimetal-Organic Frameworks: A Highly Active Catalyst for Furfuryl Alcohol Hydrogenation. ACS Catal. 2018, 8, 2121–2128. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Zhu, W.; Xiao, G. Selective Hydrogenation of Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol over Ni/γ-Al2O3 Catalysts. Res. Chem. Intermed. 2017, 43, 1179–1195. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic Conversion of Furfural to Industrial Chemicals over Supported Pt and Pd Catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hsu, C.Y.; Chen, S.S.; Ahamad, T.; Alshehri, S.M.; Tsang, D.C.W.; Wu, K.C.W. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over a Rh-Loaded Carbon Catalyst in Aqueous Solution under Mild Conditions. Sustain. Energy Fuels 2019, 4, 293–301. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal Catalysts for the Efficient Transformation of Biomass-Derived HMF and Furfural to Value Added Chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over Ni/CNTs and Bimetallic Cu[Sbnd]Ni/CNTs Catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M.A. Control over Hydrogenation Selectivity of Furfural via Tuning Exposed Facet of Ni Catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Rao, T.U.; Suchada, S.; Choi, C.; Machida, H.; Huo, Z.; Norinaga, K. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol in 2-Butanol over an Equimolar Ni-Cu-Al Catalyst Prepared by the Co-Precipitation Method. Energy Convers. Manag. 2022, 265, 115736. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Jia, X.; Du, Z.; Duan, Y.; Xu, J. Aqueous Phase Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol on Alkaline Earth Metal Modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, H.; Wang, G.; Zhao, H. Highly Dispersed Co and Ni Nanoparticles Encapsulated in N-Doped Carbon Nanotubes as Efficient Catalysts for the Reduction of Unsaturated Oxygen Compounds in Aqueous Phase. Catal. Sci. Technol. 2018, 8, 5506–5514. [Google Scholar] [CrossRef]
- Koley, P.; Rao, B.S.; Sabri, Y.M.; Bhargava, S.K.; Tardio, J.; Lingaiah, N. Selective Conversion of Furfural into Tetrahydrofurfuryl Alcohol Using a Heteropoly Acid-Based Material as a Hydrogenation Catalyst. Sustain. Energy Fuels 2020, 4, 4768–4779. [Google Scholar] [CrossRef]
- Nakajima, K.; Noma, R.; Kitano, M.; Hara, M. Titania as an Early Transition Metal Oxide with a High Density of Lewis Acid Sites Workable in Water. J. Phys. Chem. C 2013, 117, 16028–16033. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Li, H.; Sagar, T.V. TiO2-Based Water-Tolerant Acid Catalysis for Biomass-Based Fuels and Chemicals. ACS Catal. 2020, 10, 9555–9584. [Google Scholar] [CrossRef]
- Tolek, W.; Nanthasanti, N.; Pongthawornsakun, B.; Praserthdam, P.; Panpranot, J. Effects of TiO2 Structure and Co Addition as a Second Metal on Ru-Based Catalysts Supported on TiO2 for Selective Hydrogenation of Furfural to FA. Sci. Rep. 2021, 11, 9786. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Takahashi, R.; Yamada, I.; Nakanishi, K.; Sato, S. Sol-Gel Preparation of Ni/TiO2 Catalysts with Bimodal Pore Structures. Appl. Catal. A Gen. 2010, 383, 66–72. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, P.; Zhao, B.; Liu, K.; Kung, M.C.; Kung, H.H.; Chen, S.; Zhang, Z.C. Selective Hydrodeoxygenation of Guaiacol to Phenolics by Ni/Anatase TiO2 Catalyst Formed by Cross-Surface Migration of Ni and TiO2. ACS Catal. 2019, 9, 3551–3563. [Google Scholar] [CrossRef]
- Liu, K.; Yan, P.; Jiang, H.; Xia, Z.; Xu, Z.; Bai, S.; Zhang, Z.C. Silver Initiated Hydrogen Spillover on Anatase TiO2 Creates Active Sites for Selective Hydrodeoxygenation of Guaiacol. J. Catal. 2019, 369, 396–404. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Xu, Y.; Li, H.; Li, H.; Li, P.; Zhou, X. Nickel Nanoparticles Embedded in the Framework of Mesoporous TiO2: Efficient and Highly Stable Catalysts for Hydrodechlorination of Chlorobenzene. Appl. Catal. A Gen. 2012, 413–414, 350–357. [Google Scholar] [CrossRef]
- Balla, P.; Seelam, P.K.; Balaga, R.; Rajesh, R.; Perupogu, V.; Liang, T.X. Immobilized Highly Dispersed Ni Nanoparticles over Porous Carbon as an Efficient Catalyst for Selective Hydrogenation of Furfural and Levulinic Acid. J. Environ. Chem. Eng. 2021, 9, 106530. [Google Scholar] [CrossRef]
- Yin, S.; Zhu, L.; Liu, Y.; Wang, X.; Liu, Y.; Wang, S. Effect of Ni Precipitation Method on CO Methanation over Ni/TiO2 Catalysts. Chem. Res. Chin. Univ. 2018, 34, 296–301. [Google Scholar] [CrossRef]
- Tian, H.; Li, S.; Zeng, L.; Ma, H.; Gong, J. Assembly of Ordered Mesoporous Alumina-Supported Nickel Nanoparticles with High Temperature Stability for CO Methanation. Sci. China Mater. 2015, 58, 9–15. [Google Scholar] [CrossRef]
- Gao, W.; Li, W.; Xue, Z.; Pal, M.; Liu, Y.; Wang, C.; Wang, J.; Wang, S.; Wan, X.; Liu, Y.; et al. Preparation of Mesoporous TiO2-C Composites as an Advanced Ni Catalyst Support for Reduction of 4-Nitrophenol. New J. Chem. 2016, 40, 4200–4205. [Google Scholar] [CrossRef]
- Sakthivel, T.; Kumar, K.A.; Senthilselvan, J.; Jagannathan, K. Effect of Ni Dopant in TiO2 Matrix on Its Interfacial Charge Transportation and Efficiency of DSSCs. J. Mater. Sci. Mater. Electron. 2018, 29, 2228–2235. [Google Scholar] [CrossRef]
- Senthamarai, R.; Madurai Ramakrishnan, V.; Murugan, P.; Ponnusamy Munusamy, A.; Kulandhaivel, S. Synthesis and Characterization of Nickel Doped TiO2 Nanoparticles by Green Method and Its Performance as Dye-Sensitized Solar Cells Photoanodes. Int. J. Energy Res. 2022, 46, 7749–7757. [Google Scholar] [CrossRef]
- Buddee, S.; Suwanchawalit, C.; Wongnawa, S. Nickel Doped Nanorod Titanium Dioxide Photocatalyst with Enhanced Visible Light Photocatalytic Performance. Dig. J. Nanomater. Biostruct. 2017, 12, 829–839. [Google Scholar]
- Torkaman, M.; Rasuli, R.; Taran, L. Photovoltaic and Photocatalytic Performance of Anchored Oxygen-Deficient TiO2 Nanoparticles on Graphene Oxide. Results Phys. 2020, 18, 103229. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Owen, S.L.; Abrokwah, R.Y.; Kuila, D. Mesoporous Nanocrystalline TiO2 Supported Metal (Cu, Co, Ni, Pd, Zn, and Sn) Catalysts: Effect of Metal-Support Interactions on Steam Reforming of Methanol. J. Mol. Catal. A Chem. 2015, 408, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Raguram, T.; Rajni, K.S. Synthesis and Analysing the Structural, Optical, Morphological, Photocatalytic and Magnetic Properties of TiO2 and Doped (Ni and Cu) TiO2 Nanoparticles by Sol–Gel Technique. Appl. Phys. A Mater. Sci. Process. 2019, 125, 288. [Google Scholar] [CrossRef]
- Bleta, R.; Alphonse, P.; Lorenzato, L. Nanoparticle Route for the Preparation in Aqueous Medium of Mesoporous TiO2 with Controlled Porosity and Crystalline Framework. J. Phys. Chem. C 2010, 114, 2039–2048. [Google Scholar] [CrossRef]
- Yun, H.-S.; Miyazawa, K.; Honma, I.; Zhou, H.; Kuwabara, M. Synthesis of Semicrystallized Mesoporous TiO2 Thin Films Using Triblock Copolymer Templates. Mater. Sci. Eng. C 2003, 23, 487–494. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced Low-Temperature Activity of CO2 Methanation over Highly-Dispersed Ni/TiO2 Catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, D.; Wu, D. Industrially Applicable Aqueous-Phase Selective Hydrogenation of Furfural on an Efficient TiOx-Modified Ni Nanocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 13902–13914. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.; Jin, Y.; Zhu, J.; Liu, S.; Lin, J.; Li, Z. Silica/Titania Composite-Supported Ni Catalysts for CO Methanation: Effects of Ti Species on the Activity, Anti-Sintering, and Anti-Coking Properties. Appl. Catal. B Environ. 2017, 201, 561–572. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; Vandevondele, J.; Ekinci, Y.; Van Bokhoven, J.A. Catalyst Support Effects on Hydrogen Spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, P.; Zhao, B.; Zhang, Z.C. Identification of Electron-Rich Mononuclear Ni Atoms on TiO2-A Distinguished from Ni Particles on TiO2-R in Guaiacol Hydrodeoxygenation Pathways. Catal. Sci. Technol. 2021, 11, 297–311. [Google Scholar] [CrossRef]
- Huang, L.; Lv, Y.; Liu, S.; Cui, H.; Zhao, Z.; Zhao, H.; Liu, P.; Xiong, W.; Hao, F.; Luo, H. Non-Noble Metal Ni Nanoparticles Supported on Highly Dispersed TiO2-Modified Activated Carbon as an Efficient and Recyclable Catalyst for the Hydrogenation of Halogenated Aromatic Nitro Compounds under Mild Conditions. Ind. Eng. Chem. Res. 2020, 59, 1422–1435. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Liu, C.J. Structural Effect of Ni/TiO2on CO Methanation: Improved Activity and Enhanced Stability. RSC Adv. 2021, 12, 721–727. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2-x-Modified Ni Nanocatalyst with Tunable Metal-Support Interaction for Water-Gas Shift Reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Jin, X.; Sun, W.; Zhang, Q.; Ruan, K.; Cheng, Y.; Xu, H.; Xu, Z.; Li, Q. Reduced Energy Offset via Substitutional Doping for Efficient Organic/Inorganic Hybrid Solar Cells. Opt. Express 2015, 23, A444. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-Doped TiO2 Nanotubes for Wide-Range Hydrogen Sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; John, R. Impact of Ni Metal Ion Concentration in TiO2 Nanoparticles for Enhanced Photovoltaic Performance of Dye Sensitized Solar Cell. J. Mater. Sci. Mater. Electron. 2021, 32, 5295–5308. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, L.; Ke, C.; Fan, G.; Yang, L.; Li, F. Highly Efficient Catalytic Transfer Hydrogenation of Furfural over Defect-Rich Amphoteric ZrO2 with Abundant Surface Acid-Base Sites. Dalton Trans. 2021, 50, 2616–2626. [Google Scholar] [CrossRef] [PubMed]
- Parikh, J.; Srivastava, S.; Jadeja, G.C. Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol Using Supported Nickel–Cobalt Catalysts. Ind. Eng. Chem. Res. 2019, 58, 16138–16152. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, M.; Yang, J.; Shen, F.; Wang, X.; Qi, X. One-Pot Self-Assembly Synthesis of Ni-Doped Ordered Mesoporous Carbon for Quantitative Hydrogenation of Furfural to Furfuryl Alcohol. Green Chem. 2021, 23, 1861–1870. [Google Scholar] [CrossRef]
- Chen, C.; Fan, R.; Gong, W.; Zhang, H.; Wang, G.; Zhao, H. The Catalytic Behaviour in Aqueous-Phase Hydrogenation over a Renewable Ni Catalyst Derived from a Perovskite-Type Oxide. Dalton Trans. 2018, 47, 17276–17284. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Tabanelli, T.; Cavani, F.; Raspolli Galletti, A.M. Integrated Cascade Process for the Catalytic Conversion of 5-Hydroxymethylfurfural to Furanic and TetrahydrofuranicDiethers as Potential Biofuels. ChemSusChem 2022, 15, e202200241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Guo, Q.; Zhang, Y. Ni@C@CNT Catalyst Derived from CNT Doped Ni-MOF for Furfural Hydrogenation to Tetrahydrofurfuryl Alcohol. Asia-Pac. J. Chem. Eng. 2022, 17, e2739. [Google Scholar] [CrossRef]
- Su, Y.; Chen, C.; Zhu, X.; Zhang, Y.; Gong, W.; Zhang, H.; Zhao, H.; Wang, G. Carbon-Embedded Ni Nanocatalysts Derived from MOFs by a Sacrificial Template Method for Efficient Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. Dalton Trans. 2017, 46, 6358–6365. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Luo, J.; Niu, H.; Li, R.; Liang, C. Structure Evolution of Ni–Cu Bimetallic Catalysts Derived from Layered Double Hydroxides for Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. Ind. Eng. Chem. Res. 2022, 61, 12953–12965. [Google Scholar] [CrossRef]
- Sunyol, C.; English Owen, R.; González, M.D.; Salagre, P.; Cesteros, Y. Catalytic Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol Using Competitive Nickel Catalysts Supported on Mesoporous Clays. Appl. Catal. A Gen. 2021, 611, 117903. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Mironenko, R.M.; Gulyaeva, T.I.; Trenikhin, M.V.; Muromtsev, I.V.; Trubina, S.V.; Zvereva, V.V.; Likholobov, V.A. Catalysts Derived from Nickel-Containing Layered Double Hydroxides for Aqueous-Phase Furfural Hydrogenation. Catalysts 2022, 12, 598. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T.; et al. Thermally Stable Single Atom Pt/m-Al2O3 for Selective Hydrogenation and CO Oxidation. Nat. Commun. 2017, 8, 16100. [Google Scholar] [CrossRef] [PubMed]










| S.No | Catalyst | Ni Loading (%) | H2 Consumption * | N(H2)/N(Ni) * | |
|---|---|---|---|---|---|
| ICP | SEM-EDS | mmol/g | |||
| 1 | m-TiO2 | — | — | — | — |
| 2 | 2.5Ni/m-TiO2 | 2.85 | 2.82 | 0.611 | 1.26 |
| 3 | 5Ni/m-TiO2 | 5.46 | 4.78 | 1.070 | 1.15 |
| 4 | 7.5Ni/m-TiO2 | 7.97 | 7.90 | 1.624 | 1.20 |
| 5 | 10Ni/m-TiO2 | 10.97 | 10.17 | 1.931 | 1.03 |
| S.No | Catalyst | Gram of Metal (10−2) | FFA (mmol) | T # (°C) | H2 (MPa) | Rate (mol/g/h) * | Solvent | XFFA (%) | STHFA (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NiCo/SiO2-MOF | 3.10 | 41 € | 80 | 3 | 0.59 | EtOH | 99.8 | 99.1 | [11] |
| 2 | 15Ni/Al2O3 | 15.0 | 76 € | 130 | 4 | 0.49 | EtOH | 99.7 | 97.6 | [12] |
| 3 | 8.9Cu-9.8Ni/CNT | 1.87 | 6.0 | 130 | 4 | 0.25 | EtOH | 99.9 | 90.0 | [17] |
| 4 | Ni@C@CNT | 35.6 | 84.1 | 120 | 4 | 0.12 | EtOH | 99.1 | 96.7 | [58] |
| 5 | 12.7%Ni/C | 0.63 | 5.0 | 120 | 3 | 0.59 | 2-PrOH | 99.9 | 90.0 | [30] |
| 6 | Ni/MMO-CO2 | 2.80 | 6.0 | 110 | 3 | 0.14 | 2-PrOH | 99.9 | 99.9 | [18] |
| 7 | 51.1Ni/C | 15.3 | 0.31 | 120 | 1 | 0.02 | 2-PrOH | 99.9 | 99.9 | [59] |
| 8 | Cu1Ni3/MgAlO | 1.75 | 5 | 110 | 3 | 0.26 | 2-PrOH | 99.9 | 94.4 | [60] |
| 9 | NiLaO3 | 0.78 | 1.0 | 120 | 1 | 0.03 | H2O | 98.8 | 87.2 | [56] |
| 10 | Ni(40)/MgO-M | 24.0 | 15.3 | 140 | 4 | 0.05 | H2O | 99.9 | 99.9 | [61] |
| 11 | Ni@NCNTs | 1.20 | 1.0 | 100 | 4 | 0.07 | H2O | 99.9 | 99.0 | [21] |
| 12 | Ni@NiAlO-LDH | 53.3 | 60.5 | 150 | 3 | 0.05 | H2O | 99.9 | 33.0 | [62] |
| 13 | 10Ni-Ba-Al2O3 | 2.00 | 5.2 | 140 | 4 | 0.20 | H2O | 99.0 | 99.0 | [20] |
| 14 | 7.5Ni/m-TiO2 | 0.24 | 1.0 | 100 | 2 | 0.36 | H2O | 99.9 | 93.2 | PW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaga, R.; Ramineni, K.; Zhang, X.; Yan, P.; Marri, M.R.; Perupogu, V.; Zhang, Z.C. Spillover Hydrogen on Electron-Rich Ni/m-TiO2 for Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. Catalysts 2022, 12, 1286. https://doi.org/10.3390/catal12101286
Balaga R, Ramineni K, Zhang X, Yan P, Marri MR, Perupogu V, Zhang ZC. Spillover Hydrogen on Electron-Rich Ni/m-TiO2 for Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. Catalysts. 2022; 12(10):1286. https://doi.org/10.3390/catal12101286
Chicago/Turabian StyleBalaga, Ravi, Kishore Ramineni, Xiaoqiang Zhang, Peifang Yan, Mahender Reddy Marri, Vijayanand Perupogu, and Zongchao Conrad Zhang. 2022. "Spillover Hydrogen on Electron-Rich Ni/m-TiO2 for Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol" Catalysts 12, no. 10: 1286. https://doi.org/10.3390/catal12101286