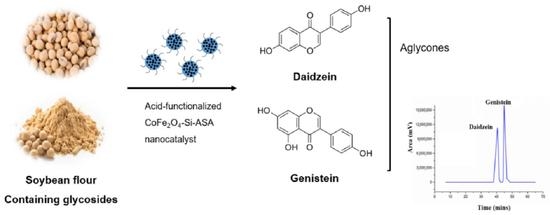
Production of Therapeutically Significant Genistein and Daidzein Compounds from Soybean Glycosides Using Magnetic Nanocatalyst: A Novel Approach
Abstract
:Highlights
- Production of therapeutical aglycone compounds was attempted using a nanocatalyst.
- The maximum amount of diadzein (8.91 g/L) and genistein (12.0 g/L) was generated at 80 °C in 3 h with yields of 0.590 and 0.621 g/g substrate, respectively..
- Reused nanocatalyst demonstrated ~35% catalytic efficiency even after third recycle.
- Nanocatalyst can be a better substitute for costly enzymes in aglycone production.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis and Functionalization of Nanoparticles
2.2.1. Synthesis of Magnetic CoFe2O4 Nanoparticles (CoFe2O4 MNPs)
2.2.2. Synthesis of Silica Coated CoFe2O4 Nanoparticles (CoFe2O4–Si MNPs)
2.2.3. Acid Functionalization of CoFe2O4-Si MNPs Using Alkylsulfonic Acid (CoFe2O4-Si-ASA)
2.3. Characterization of Synthesized Nanoparticles
2.4. Microbial Strain, Medium, and β-Glucosidase Enzyme Production
2.5. Hydrolysis of Soybean-Derived Glycosides Using Acid-Functionalized CoFe2O4-Si-ASA Nanocatalyst and β-Glucosidase Enzyme
2.6. Recovery and Reuse of CoFe2O4-Si-ASA Nanocatalyst for Hydrolysis Experiments
2.7. Analytical Methods
3. Results and Discussion
3.1. Synthesis and Characterization of All Synthesized Nanoparticles
3.2. β-Glucosidase Enzyme Production by Solid-State Fermentation
3.3. Comparative Studies on Hydrolysis of Soybean-Derived Glycosides Using CoFe2O4-Si-ASA Nanocatalyst and β-Glucosidase Enzyme
3.3.1. Hydrolysis of Soybean-Derived Glycosides Using CoFe2O4-Si-ASA Nanocatalyst
3.3.2. Hydrolysis of Soybean-Derived Glycosides Using β-Glucosidase Enzymes
3.4. Recycling of Used CoFe2O4-Si-ASA Nanocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albertazzi, P.; Purdie, D.W. The nature and utility of the phytoestrogens: A review of the evidence. Maturitas 2002, 42, 173–185. [Google Scholar] [CrossRef]
- Nemitz, M.C.; Picada, J.N.; da Silva, J.; Garcia, A.L.H.; Papke, D.K.; Grivicich, I.; Teixeira, H.F. Determination of the main impurities formed after acid hydrolysis of soybean extracts and the in vitro mutagenicity and genotoxicity studies of 5-ethoxymethyl-2-furfural. J. Pharm. Biomed. Anal. 2016, 129, 427–432. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef]
- He, F.J.; Chen, J.Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161. [Google Scholar] [CrossRef]
- Halabalaki, M.; Alexi, X.; Aligiannis, N.; Lambrinidis, G.; Pratsinis, H.; Florentin, I.; Mitakou, S.; Mikros, E.; Skaltsounis, A.-L.; Alexis, M.N.; et al. Estrogenic activity of isoflavonoids from Onobrychis ebenoides. Planta Med. 2006, 72, 488–493. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Hillman, G.G.; Singh-Gupta, V.; Al-Bashir, A.K.; Yunker, C.K.; Joiner, M.C.; Sarkar, F.H.; Abrams, J.; Mark Haacke, E. Monitoring sunitinib-induced vascular effects to optimize radiotherapy combined with soy isoflavones in murine xenograft tumor. Transl. Oncol. 2011, 4, 110–121. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Zhang, B.; Liu, R.; Marcone, M.F.; Li, X.; Tsao, R. 5-Hydroxymethyl-2-furfural and derivatives formed during acid hydrolysis of conjugated and bound phenolics in plant foods and the effects on phenolic content and antioxidant capacity. J. Agric. Food Chem. 2014, 62, 4754–4761. [Google Scholar] [CrossRef]
- Nemitz, M.C.; Moraes, R.C.; Koester, L.S.; Bassani, V.L.; von Poser, G.L.; Teixeira, H.F. Bioactive soy isoflavones: Extraction and purification procedures, potential dermal use and nanotechnology-based delivery systems. Phytochem. Rev. 2015, 14, 849–869. [Google Scholar] [CrossRef]
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Evaluation of glucosidases of Aspergillus niger strain comparing with other glucosidases in transformation of ginsenoside Rb1 to ginsenosides Rg3. J. Ginseng Res. 2014, 38, 47–51. [Google Scholar] [CrossRef]
- Feng, C.; Jin, S.; Xia, X.X.; Guan, Y.; Luo, M.; Zu, Y.G.; Fu, Y.J. Effective bioconversion of sophoricoside to genistein from Fructus sophorae using immobilized Aspergillus niger and yeast. World J. Microbiol. Biotechnol. 2015, 31, 187–197. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S. Production of pharmaceutically important genistein and daidzein from soybean flour extract by using β-glucosidase derived from Penicillium janthinellum NCIM 1171. Process Biochem. 2020, 97, 183–190. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Zheng, A.; Xiao, F.S.; Dai, S. Hydrophobic solid acids and their catalytic applications in green and sustainable chemistry. ACS Catal. 2018, 8, 372–391. [Google Scholar] [CrossRef]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid acid catalyst based on single-layer α-zirconium phosphate nanosheets for biodiesel production via esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef]
- Peña, L.; Xu, F.; Hohn, K.L.; Li, J.; Wang, D. Propyl-sulfonic acid functionalized nanoparticles as catalyst for pretreatment of corn stover. J. Biomater. Nanobiotechnol. 2014, 5, 8–16. [Google Scholar] [CrossRef]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Peña, L.; Ikenberry, M.; Ware, B.; Hohn, K.L.; Boyle, D.; Sun, X.S.; Wang, D. Cellobiose hydrolysis using acid-functionalized nanoparticles. Biotechnol. Bioprocess Eng. 2011, 16, 1214–1222. [Google Scholar] [CrossRef]
- Carlier, S.; Hermans, S. Highly efficient and recyclable catalysts for cellobiose hydrolysis: Systematic comparison of carbon nanomaterials functionalized with benzyl sulfonic acids. Front. Chem. 2020, 8, 347. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Deshmukh, A.R.; Kim, B.S. Cellulase mimicking nanomaterial-assisted cellulose hydrolysis for enhanced bioethanol fermentation: An emerging sustainable approach. Green Chem. 2021, 23, 5064–5081. [Google Scholar] [CrossRef]
- Wang, D.; Ikenberry, M.; Pe, L.; Hohn, K.L. Acid-functionalized nanoparticles for pretreatment of wheat straw. J. Biomater. Nanobiotechnol. 2012, 3, 342–352. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; de Souza Melo, Y.C.; da Silva, S.S. Acid-functionalized magnetic nanocatalysts mediated pretreatment of sugarcane straw: An eco-friendly and cost-effective approach. Cellulose 2020, 27, 7067–7078. [Google Scholar] [CrossRef]
- Rajkumari, K.; Kalita, J.; Das, D.; Rokhum, L. Magnetic Fe3O4@ silica sulfuric acid nanoparticles promoted regioselective protection/deprotection of alcohols with dihydropyran under solvent-free conditions. RSC Adv. 2017, 7, 56559–56565. [Google Scholar] [CrossRef]
- Melero, J.A.; Stucky, G.D.; van Grieken, R.; Morales, G. Direct syntheses of ordered SBA-15 mesoporous materials containing arenesulfonic acid groups. J. Mater. Chem. 2002, 12, 1664–1670. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef]
- Maharjan, A.; Singhvi, M.; Kim, B.S. Biosynthesis of a therapeutically important nicotinamide mononucleotide through a phosphoribosyl pyrophosphate synthetase 1 and 2 engineered strain of Escherichia Coli. ACS Synth. Biol. 2021, 10, 3055–3065. [Google Scholar] [CrossRef]
- Tanuraghaj, H.M.; Farahi, M. Preparation, characterization and catalytic application of nano-Fe3O4@ SiO2@(CH2)3OCO2Na as a novel basic magnetic nanocatalyst for the synthesis of new pyranocoumarin derivatives. RSC Adv. 2018, 8, 27818–27824. [Google Scholar] [CrossRef] [Green Version]
- Safari, J.; Zarnegar, Z. A magnetic nanoparticle-supported sulfuric acid as a highly efficient and reusable catalyst for rapid synthesis of amidoalkyl naphthols. J. Mol. Catal. A Chem. 2013, 379, 269–276. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.; Hueso, J.L.; Vallet-Regí, M. Synthesis and characterization of zwitterionic SBA-15 nanostructured materials. Chem. Mater. 2010, 22, 6459–6466. [Google Scholar] [CrossRef]
- Alvaro, M.; Corma, A.; Das, D.; Fornés, V.; García, H. “Nafion”-functionalized mesoporous MCM-41 silica shows high activity and selectivity for carboxylic acid esterification and Friedel–Crafts acylation reactions. J. Catal. 2005, 231, 48–55. [Google Scholar] [CrossRef]
- Ramanathan, G.; Banupriya, S.; Abirami, D. Production and optimization of cellulase from Fusarium oxysporum by submerged fermentation. J. Sci. Ind. Res. 2010, 69, 454–459. [Google Scholar]
- De Almeida, M.N.; Falkoski, D.L.; Guimarães, V.M.; de Rezende, S.T. Study of gamba grass as carbon source for cellulase production by Fusarium verticillioides and its application on sugarcane bagasse saccharification. Ind. Crops Prod. 2019, 133, 33–43. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Hong, J. A simple method for beta-glucosidase immobilization and its application in soybean isoflavone glycosides hydrolysis. Biotechnol. Bioprocess Eng. 2018, 23, 39–48. [Google Scholar] [CrossRef]
- Mei, J.; Chen, X.; Liu, J.; Yi, Y.; Zhang, Y.; Ying, G. A biotransformation process for production of genistein from sophoricoside by a strain of Rhizopus oryza. Sci. Rep. 2019, 9, 6564. [Google Scholar] [CrossRef]
- Doan, D.T.; Luu, D.P.; Nguyen, T.D.; Hoang Thi, B.; Pham Thi, H.M.; Do, H.N.; Luu, V.H.; Pham, T.D.; Than, V.T.; Thi, H.H.P.; et al. Isolation of Penicillium citrinum from roots of Clerodendron cyrtophyllum and application in biosynthesis of aglycone isoflavones from soybean waste fermentation. Foods 2019, 8, 554. [Google Scholar] [CrossRef]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid-functionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]







| Synthesized Nanoparticles | Atomic Weight Percentage (%) a | Acid Capacity (mM H+/g) b | |||||
|---|---|---|---|---|---|---|---|
| C | O | Fe | Co | Si | S | ||
| CoFe2O4 | 34.19 | 27.20 | 15.13 | 23.47 | - | - | - |
| CoFe2O4-Si | 37.16 | 53.01 | 1.13 | 1.12 | 7.59 | - | - |
| CoFe2O4-Si-ASA | 51.23 | 38.69 | 0.18 | 0.21 | 4.64 | 5.05 | 0.92 |
| Fermentation Time (Days) | β-Glucosidase (IU/g) | Protein (mg/g) |
|---|---|---|
| 2nd day | 0.453 ± 0.021 | 0.153 ± 0.0082 |
| 4th day | 1.858 ± 0.101 | 0.858 ± 0.049 |
| 6th day | 1.959 ± 0.098 | 0.895 ± 0.052 |
| 8th day | 2.052 ± 0.111 | 0.782 ± 0.043 |
| Catalyst Used | Glycosides (g/L) | Aglycones (g/L) | ||
|---|---|---|---|---|
| Daidzin | Genistin | Daidzein | Genistein | |
| CoFe2O4-Si-ASA (80 °C for 3 h) | 15.1 ± 0.51 | 19.3 ± 0.68 | 8.91 ± 0.45 (59.0%) | 12.0 ± 0.48 (62.2%) |
| CoFe2O4-Si-ASA (120 °C at 15 psi for 1 h) | 15.5 ± 0.68 | 18.7 ± 0.99 | 10.7 ± 0.41 (69.2%) | 13.7 ± 0.51 (73.3%) |
| β-glucosidase enzyme (60 °C for 2 h) | 14.8 ± 0.81 | 17.9 ± 0.91 | 12.1 ± 0.72 (81.8%) | 15.6 ± 0.62 (87.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhvi, M.; Kim, M.; Kim, B.-S. Production of Therapeutically Significant Genistein and Daidzein Compounds from Soybean Glycosides Using Magnetic Nanocatalyst: A Novel Approach. Catalysts 2022, 12, 1107. https://doi.org/10.3390/catal12101107
Singhvi M, Kim M, Kim B-S. Production of Therapeutically Significant Genistein and Daidzein Compounds from Soybean Glycosides Using Magnetic Nanocatalyst: A Novel Approach. Catalysts. 2022; 12(10):1107. https://doi.org/10.3390/catal12101107
Chicago/Turabian StyleSinghvi, Mamata, Minseong Kim, and Beom-Soo Kim. 2022. "Production of Therapeutically Significant Genistein and Daidzein Compounds from Soybean Glycosides Using Magnetic Nanocatalyst: A Novel Approach" Catalysts 12, no. 10: 1107. https://doi.org/10.3390/catal12101107