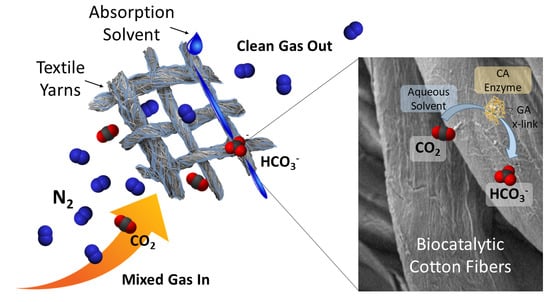
Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture
Abstract
:1. Introduction
| CA Forms | Solvent and Testing Systems | Biocatalyst Performance Results | Reference |
|---|---|---|---|
| Free CA at 0–3 g/L | 20 wt% K2CO3 (1.8 M); Stirred tank reactor and packed-bed column | High CA dose is able to boost CO2 absorption rate of 20 wt% K2CO3 comparable to that of 5 M (~30 wt%) MEA | Zhang et al. [24] |
| Free CA at 0–2.4 g/L | 1–4 M MDEA; 0.3–0.6 M Na2CO3; Stirred cell contactor | Reduce the absorber size required for kinetically limited low energy solvents to the size achieved by commercial scale MEA CO2 capture systems | Penders-van Elk et al. [65] |
| Free CA at 0–2.5 g/L CA | 23.5 wt% K2CO3 (2.2 M); Bench-scale unit packed with ceramic Raschig rings and with vacuum stripping | 4.6-fold increase in CO2 capture efficiency at CA concentration of 2.5 g/L CA; a minimum of 10% daily (every 7 h run time) enzyme replenishment was required to maintain a consistent CO2 capture efficiency | Qi et al. [12,66] |
| Free CA at >0.3 g/L | 2–3 M K2CO3; stirred cell apparatus | 5–8-fold increase in CO2 absorption rate with >0.3 g/L precipitated enzyme aggregates acting as biocatalysts | Pierce et al. [15] |
| Immobilized CA “in vivo” anchored on cell membrane | 0.5 M Na2CO3/0.5 M NaHCO3 buffer; stirred cell apparatus | Enhancement factor of 1.3–2.4 with dispersed CA-anchored cell debris acting as biocatalyst | Fabbricino et al. [67] |
| Immobilized CA entrapped in organosilica matrix coated on stainless steel structured packing | 20 wt% K2CO3 (1.8 M)and a proprietary non-volatile alkaline salt solvent; packed column reactors from lab- to field- scale | 6 to 7-fold rate enhancement for immobilized CA packing over the no-enzyme packings and a biocatalyst half-life of 539 days determined from 3500 h continuous field test | Reardon et al. [68,69] |
| Immobilized CA entrapped in organosilica micro-particles | 30 wt% MDEA (2.5 M); counter-current packed column | Immobilized CA in the form of buoyant micro-particles that can be easily separated and reused, yielded a 6-fold enhancement in CO2 absorption | Leimbrink et al. [70,71] |
| Immobilized CA entrapped in chitosan matrix coated on textile structured packing | 10 wt% K2CO3 (0.8 M); Lab-scale packed column | 2-fold and 14.5-fold enhancement in CO2 capture efficiency over no-enzyme textile control packing and over conventional glass Raschig rings, respectively; 66% performance retention after 5 repeated test-wash-storage cycles; 76.5% performance retention after a 120 h continuous solvent flow scrubber test | Shen et al. [72] |
| Immobilized CA covalently attached on textile structured packing | 5–30 wt% K2CO3 (0.4–2.8 M); 5–10 wt% DMG (0.4–0.8 M); 5 wt% MDEA (0.4 M); Simulated seawater (3.5 wt% solute); Lab-scale packed column | 2.9-fold and 19.1-fold enhancement in CO2 capture efficiency over no-enzyme textile control packing and conventional glass Raschig rings, respectively; 100% performance retention after 10 repeated test-wash-storage cycles including a 100 h incubation in in 10 wt% K2CO3 at 45 °C; steady (~100%) performance retention over a 500 h continuous solvent flow scrubber test; 85.7% performance retention after 1 year dry storage | This study |
2. Results and Discussions
2.1. Surface Chemistry
2.2. Surface Morphology
2.3. Liquid Transport Property
2.4. Assay Scale Longevity Tests
2.5. Lab-Scale CO2 Gas Scrubber Test
2.6. Effects of Solvent and Gas Flow Rates
2.7. Effect of Solvent Types and Concentrations
2.8. Longevity of Enzyme Immobilized Textile Packing
2.9. Versatile Application Conditions
3. Materials and Methods
3.1. Materials, Chemicals, and Enzymes
3.2. Instrumental Characterizations
3.3. Enzyme Immobilization on Textile Support Materials
3.4. Assay-Scale Longevity Tests
3.4.1. Stress Testing by Rotisserie Mixing
3.4.2. Orbital Shaker Stress Test
3.5. Laboratory Gas Scrubber Test
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, B.; Braswell, B.H. The Lifetime of Excess Atmospheric Carbon Dioxide. Global Biogeochem. Cycles 1994, 8, 23–38. [Google Scholar] [CrossRef]
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-Resolution Carbon Dioxide Concentration Record 650,000-800,000 Years before Present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Lenton, T.; Rockström, J.; Gaffney, O.; Rahmstorf, S.; Richardson, K.; Steffen, W.; Shellnhuber, H.J. Climate Tipping Points—Too Risky to Bet Against. Nature 2019, 575, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Proceedings of the IPCC, Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- IEA Greenhouse Gas Emissions from Energy: Overview. Available online: https://www.iea.org/reports/greenhouse-gas-emissions-from-energy-overview (accessed on 9 August 2021).
- National Academies of Sciences Engineering and Medicine. Accelerating Decarbonization of the U.S. Energy System; The National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-68292-3. [Google Scholar]
- Bettenhausen, C.A. Carbon Capture’s Steep Climb. Chem. Eng. News 2021, 99, 28–35. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.; He, C.; Liang, Z. Experimental Evaluation of Highly Efficient Primary and Secondary Amines with Lower Energy by a Novel Method for Post-Combustion CO2 Capture. Appl. Energy 2019, 233–234, 443–452. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current Status of CO2 Chemical Absorption Research Applied to CCS: Towards Full Deployment at Industrial Scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Qi, G.; Liu, K.; House, A.; Salmon, S.; Ambedkar, B.; Frimpong, R.A.; Remias, J.E.; Liu, K. Laboratory to Bench-Scale Evaluation of an Integrated CO2 Capture System Using a Thermostable Carbonic Anhydrase Promoted K2CO3 Solvent with Low Temperature Vacuum Stripping. Appl. Energy 2018, 209, 180–189. [Google Scholar] [CrossRef]
- Gupta, M.; Da Silva, E.F.; Hartono, A.; Svendsen, H.F. Theoretical Study of Differential Enthalpy of Absorption of CO2 with MEA and MDEA as a Function of Temperature. J. Phys. Chem. B 2013, 117, 9457–9468. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A. Minimising the Regeneration Heat Duty of Post-Combustion CO2 Capture by Wet Chemical Absorption: The Misguided Focus on Low Heat of Absorption Solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Peirce, S.; Perfetto, R.; Russo, M.E.; Capasso, C.; Rossi, M.; Salatino, P.; Marzocchella, A. Characterization of Technical Grade Carbonic Anhydrase as Biocatalyst for CO2 Capture in Potassium Carbonate Solutions. Greenh. Gases Sci. Technol. 2018, 8, 279–291. [Google Scholar] [CrossRef]
- Russo, M.E.; Olivieri, G.; Marzocchella, A.; Salatino, P.; Caramuscio, P.; Cavaleiro, C. Post-Combustion Carbon Capture Mediated by Carbonic Anhydrase. Sep. Purif. Technol. 2013, 107, 331–339. [Google Scholar] [CrossRef]
- Russo, M.E.; Olivieri, G.; Capasso, C.; De Luca, V.; Marzocchella, A.; Salatino, P.; Rossi, M. Kinetic Study of a Novel Thermo-Stable α-Carbonic Anhydrase for Biomimetic CO2 Capture. Enzyme Microb. Technol. 2013, 53, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Long, N.; Lee, J.; Koo, K.-K.; Luis, P.; Lee, M. Recent Progress and Novel Applications in Enzymatic Conversion of Carbon Dioxide. Energies 2017, 10, 473. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Ng, I.S. The Prospective and Potential of Carbonic Anhydrase for Carbon Dioxide Sequestration: A Critical Review. Process Biochem. 2019, 87, 55–65. [Google Scholar] [CrossRef]
- Salmon, S.; House, A. Enzyme-Catalyzed Solvents for CO2 Separation. In Novel Materials for Carbon Dioxide Mitigation Technology; Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 23–86. [Google Scholar]
- Fitzgerald, D.; Vidal, R.; Russell, T.; Babcock, D.; Freeman, C.; Bearden, M.; Whyatt, G.; Liu, K.; Frimpong, R.; Lu, K.; et al. Preliminary Environmental, Health and Safety Risk Assessment on the Integration of a Process Utilizing Low-Energy Solvents for Carbon Dioxide Capture Enabled by a Combination of Enzymes and Vacuum Regeneration with a Subcritical Pc Power Plant. Available online: https://www.osti.gov/biblio/1212663 (accessed on 30 August 2022).
- Borhani, T.N.; Wang, M. Role of Solvents in CO2 Capture Processes: The Review of Selection and Design Methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Luis, P. Use of Monoethanolamine (MEA) for CO2 Capture in a Global Scenario: Consequences and Alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y. Kinetic Performance of CO2 Absorption into a Potassium Carbonate Solution Promoted with the Enzyme Carbonic Anhydrase: Comparison with a Monoethanolamine Solution. Chem. Eng. J. 2015, 279, 335–343. [Google Scholar] [CrossRef] [Green Version]
- James, R., III; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N.; Zoelle, A. Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity; NETL-PUB-22638; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2019. [CrossRef]
- Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. Immobilization of Carbonic Anhydrase for CO2 Capture and Utilization. Appl. Microbiol. Biotechnol. 2022, 106, 3419–3430. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Walde, P. Immobilized Carbonic Anhydrase: Preparation, Characteristics and Biotechnological Applications. World J. Microbiol. Biotechnol. 2018, 34, 151. [Google Scholar] [CrossRef] [PubMed]
- Shekh, A.Y.; Krishnamurthi, K.; Mudliar, S.N.; Yadav, R.R.; Fulke, A.B.; Devi, S.S.; Chakrabarti, T. Recent Advancements in Carbonic Anhydrase-Driven Processes for CO2 Sequestration: Minireview. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1419–1440. [Google Scholar] [CrossRef]
- González, J.M.; Fisher, S.Z. Carbonic Anhydrases in Industrial Applications. In Sub-Cellular Biochemistry; Frost, S.C., McKenna, R., Eds.; Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2014; Volume 75, pp. 405–426. [Google Scholar]
- Boone, C.D.; McKenna, R. Engineered Mammalian Carbonic Anhydrases for CO2 Capture. In Carbonic Anhydrases as Biocatalysts; Carbonic Anhydrases as Biocatalysts; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–309. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the One-Step Immobilization-Purification of Enzymes as Industrial Biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, S.; Fang, X.; Salmon, S. Advances in 3D Gel Printing for Enzyme Immobilization. Gels 2022, 8, 460. [Google Scholar] [CrossRef]
- Badjić, J.D.; Kostić, N.M. Effects of Encapsulation in Sol-Gel Silica Glass on Esterase Activity, Conformational Stability, and Unfolding of Bovine Carbonic Anhydrase II. Chem. Mater. 1999, 11, 3671–3679. [Google Scholar] [CrossRef]
- Vinoba, M.; Lim, K.S.; Lee, S.H.; Jeong, S.K.; Alagar, M. Immobilization of Human Carbonic Anhydrase on Gold Nanoparticles Assembled onto Amine/Thiol-Functionalized Mesoporous SBA-15 for Biomimetic Sequestration of CO2. Langmuir 2011, 27, 6227–6234. [Google Scholar] [CrossRef]
- Peirce, S.; Russo, M.E.; Perfetto, R.; Capasso, C.; Rossi, M.; Fernandez-Lafuente, R.; Salatino, P.; Marzocchella, A. Kinetic Characterization of Carbonic Anhydrase Immobilized on Magnetic Nanoparticles as Biocatalyst for CO2 Capture. Biochem. Eng. J. 2018, 138, 1–11. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Jeong, S.K.; Nam, S.C.; Yoon, Y. Carbonic Anhydrase Immobilized on Encapsulated Magnetic Nanoparticles for CO2 Sequestration. Chem. A Eur. J. 2012, 18, 12028–12034. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Jeong, S.K.; Yoon, Y.I.I.; Nam, S.C. Immobilization of Carbonic Anhydrase on Spherical SBA-15 for Hydration and Sequestration of CO2. Colloids Surf. B Biointerf. 2012, 90, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, Y.; Ye, X. Catalytic Behavior of Carbonic Anhydrase Enzyme Immobilized onto Nonporous Silica Nanoparticles for Enhancing CO2 Absorption into a Carbonate Solution. Int. J. Greenh. Gas Control 2013, 13, 17–25. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, H.; Lu, Y. Enhanced Stability and Chemical Resistance of a New Nanoscale Biocatalyst for Accelerating CO2 Absorption into a Carbonate Solution. Environ. Sci. Technol. 2013, 47, 13882–13888. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Yang, Z.; Pan, F.; Zhou, Z.; Jing, G. Immobilization of Carbonic Anhydrase on Carboxyl-Functionalized Ferroferric Oxide for CO2 Capture. Int. J. Biol. Macromol. 2015, 79, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dong, G.; Xiao, B.; Malassigne, C.; Chen, V. Preparation of Titania Based Biocatalytic Nanoparticles and Membranes for CO2 Conversion. J. Mater. Chem. A 2015, 3, 3332–3342. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Kumar, M.; Singh, A.; Singh, M.P.; Puri, S.K.; Ramakumar, S.S.V. Accelerated CO2 Capture in Hybrid Solvent Using Co-Immobilized Enzyme/Complex on a Hetero-Functionalized Support. J. CO2 Util. 2017, 21, 77–81. [Google Scholar] [CrossRef]
- Lopes, J.H.; Guilhou, M.; Marelli, B.; Omenetto, F.G.; Kaplan, D.L.; Barralet, J.E.; Merle, G. Silk Fibroin Hydroxyapatite Composite Thermal Stabilisation of Carbonic Anhydrase. J. Mater. Chem. A 2015, 3, 19282–19287. [Google Scholar] [CrossRef]
- Trachtenberg, M.C.; Cowan, R.M.; Smith, D.A.; Horazak, D.A.; Jensen, M.D.; Laumb, J.D.; Vucelic, A.P.; Chen, H.; Wang, L.; Wu, X. Membrane-Based, Enzyme-Facilitated, Efficient Carbon Dioxide Capture. Energy Procedia 2009, 1, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-T.; Zhang, L.; Chen, H.-L.; Zhang, H.-M. Selective Separation of Low Concentration CO2 Using Hydrogel Immobilized CA Enzyme Based Hollow Fiber Membrane Reactors. Chem. Eng. Sci. 2010, 65, 3199–3207. [Google Scholar] [CrossRef]
- Arazawa, D.T.; Oh, H.I.; Ye, S.H.; Johnson, C.A.; Woolley, J.R.; Wagner, W.R.; Federspiel, W.J. Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO2 Removal from Blood. J. Memb. Sci. 2012, 403–404, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, J.D.; Arazawa, D.T.; Ye, S.H.; Shankarraman, V.; Wagner, W.R.; Federspiel, W.J. Carbonic Anhydrase Immobilized on Hollow Fiber Membranes Using Glutaraldehyde Activated Chitosan for Artificial Lung Applications. J. Mater. Sci. Mater. Med. 2013, 24, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Arazawa, D.T.; Kimmel, J.D.; Finn, M.C.; Federspiel, W.J. Acidic Sweep Gas with Carbonic Anhydrase Coated Hollow Fiber Membranes Synergistically Accelerates CO2 Removal from Blood. Acta Biomater. 2015, 25, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.K.J.; Stevens, G.W.; Caruso, F.; Kentish, S.E. In Situ Layer-by-Layer Assembled Carbonic Anhydrase-Coated Hollow Fiber Membrane Contactor for Rapid CO2 Absorption. J. Memb. Sci. 2016, 514, 556–565. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Chew, N.G.P.; Malde, C.; Wang, R. Biocatalytic PVDF Composite Hollow Fiber Membranes for CO2 Removal in Gas-Liquid Membrane Contactor. J. Memb. Sci. 2019, 572, 532–544. [Google Scholar] [CrossRef]
- Hou, J.; Ji, C.; Dong, G.; Xiao, B.; Ye, Y.; Chen, V. Biocatalytic Janus Membranes for CO2 Removal Utilizing Carbonic Anhydrase. J. Mater. Chem. A 2015, 3, 17032–17041. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Matrix Molecular Weight Cut-off for Encapsulation of Carbonic Anhydrase in Polyelectrolyte Beads. J. Biomater. Sci. Polym. Ed. 2002, 13, 1175–1187. [Google Scholar] [CrossRef]
- Yadav, R.R.; Mudliar, S.N.; Shekh, A.Y.; Fulke, A.B.; Devi, S.S.; Krishnamurthi, K.; Juwarkar, A.; Chakrabarti, T. Immobilization of Carbonic Anhydrase in Alginate and Its Influence on Transformation of CO2 to Calcite. Process Biochem. 2012, 47, 585–590. [Google Scholar] [CrossRef]
- Oviya, M.; Giri, S.S.; Sukumaran, V.; Natarajan, P. Immobilization of Carbonic Anhydrase Enzyme Purified from Bacillus Subtilis VSG-4 and Its Application as CO2 Sequesterer. Prep. Biochem. Biotechnol. 2012, 42, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, S.; Nemat-Gorgani, M. Partial Unfolding of Carbonic Anhydrase Provides a Method for Its Immobilization on Hydrophobic Adsorbents and Protects It against Irreversible Thermoinactivation. Enzyme Microb. Technol. 2003, 33, 179–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Sun, G.; Tang, Q.; Bian, H. Enzymatic Properties of Immobilized Carbonic Anhydrase and the Biocatalyst for Promoting CO2 Capture in Vertical Reactor. Int. J. Greenh. Gas Control 2016, 49, 290–296. [Google Scholar] [CrossRef]
- Ozdemir, E. Biomimetic CO2 Sequestration: 1. Immobilization of Carbonic Anhydrase within Polyurethane Foam. Energy Fuels 2009, 23, 5725–5730. [Google Scholar] [CrossRef]
- Migliardini, F.; De Luca, V.; Carginale, V.; Rossi, M.; Corbo, P.; Supuran, C.T.; Capasso, C. Biomimetic CO2 Capture Using a Highly Thermostable Bacterial α-Carbonic Anhydrase Immobilized on a Polyurethane Foam. J. Enzyme Inhib. Med. Chem. 2014, 29, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.C.; Sambudi, N.S.; Park, S.B.; Lee, J.H.; Han, J.-I. Immobilization of Carbonic Anhydrase on Modified Electrospun Poly(Lactic Acid) Membranes: Quest for Optimum Biocatalytic Performance. Catal. Lett. 2015, 145, 519–526. [Google Scholar] [CrossRef]
- Ren, S.; Feng, Y.; Wen, H.; Li, C.; Sun, B.; Cui, J.; Jia, S. Immobilized Carbonic Anhydrase on Mesoporous Cruciate Flower-like Metal Organic Framework for Promoting CO2 Sequestration. Int. J. Biol. Macromol. 2018, 117, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, C.; Tan, Z.; Hou, Y.; Jia, S.; Cui, J. Carbonic Anhydrase@ZIF-8 Hydrogel Composite Membrane with Improved Recycling and Stability for Efficient CO2 Capture. J. Agric. Food Chem. 2019, 67, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Russo, M.E.; De Luca, V.; Capasso, C.; Rossi, M.; Olivieri, G.; Salatino, P.; Marzocchella, A. Immobilization of Carbonic Anhydrase for Biomimetic CO2 Capture in a Slurry Absorber as Cross-Linked Enzyme Aggregates (CLEA). Chem. Eng. Trans. 2015, 43, 259–264. [Google Scholar] [CrossRef]
- Penders-van Elk, N.J.M.C.; Hamborg, E.S.; Huttenhuis, P.J.G.; Fradette, S.; Carley, J.A.; Versteeg, G.F. Kinetics of Absorption of Carbon Dioxide in Aqueous Amine and Carbonate Solutions with Carbonic Anhydrase. Int. J. Greenh. Gas Control 2013, 12, 259–268. [Google Scholar] [CrossRef]
- Qi, G.; Liu, K.; Frimpong, R.A.; House, A.; Salmon, S.; Liu, K. Integrated Bench-Scale Parametric Study on CO2 Capture Using a Carbonic Anhydrase Promoted K2CO3 Solvent with Low Temperature Vacuum Stripping. Ind. Eng. Chem. Res. 2016, 55, 12452–12459. [Google Scholar] [CrossRef]
- Fabbricino, S.; Del Prete, S.; Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. In Vivo Immobilized Carbonic Anhydrase and Its Effect on the Enhancement of CO2 Absorption Rate. J. Biotechnol. 2021, 336, 41–49. [Google Scholar] [CrossRef]
- Reardon, J.; Bucholz, T.; Hulvey, M.; Tuttle, J.; Shaffer, A.; Pulvirenti, D.; Weber, L.; Killian, K.; Zaks, A. Low Energy CO2 Capture Enabled by Biocatalyst Delivery System. Energy Procedia 2014, 63, 301–321. [Google Scholar] [CrossRef]
- Bucholz, T.L.; Hulvey, M.K.; Reardon, J.P.; Rambo, B.M.; Pulvirenti, D.C.; Weber, L.E.; Zaks, A. Development of an Organosilica Coating Containing Carbonic Anhydrase for Applications in CO2 Capture. In Novel Materials for Carbon Dioxide Mitigation Technology; Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 117–147. [Google Scholar]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Górak, A.; Skiborowski, M. Enzymatic Reactive Absorption of CO2 in MDEA by Means of an Innovative Biocatalyst Delivery System. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Zaks, A.; Reardon, J.; Bucholz, T.; Hulvey, M. Process for Carbon Dioxide Capture with Biocatalyst Recovery System. WO/2016/057918, 14 April 2016. [Google Scholar]
- Shen, J.; Yuan, Y.; Salmon, S. Carbonic Anhydrase Immobilized on Textile Structured Packing Using Chitosan Entrapment for CO2 Capture. ACS Sustain. Chem. Eng. 2022, 10, 7772–7785. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Bilheux, H.; Salmon, S. Biocatalytic Yarn for Peroxide Decomposition with Controlled Liquid Transport. Adv. Mater. Interf. 2021, 8, 2002104. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- López-Gallego, F.; Guisán, J.M.; Betancor, L. Glutaraldehyde-Mediated Protein Immobilization. In Immobilization of Enzymes and Cells: Third Edition; Guisan, J.M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 33–41. ISBN 978-1-62703-549-1. [Google Scholar]
- Götz, A.; Nikzad-Langerodi, R.; Staedler, Y.; Bellaire, A.; Saukel, J. Apparent Penetration Depth in Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Spectroscopy of Allium cepa L. Epidermis and Cuticle. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 224, 117460. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.P.; Peng, F.; Bian, J.; Yuan, T.Q.; Xu, F.; Sun, R.C. Microwave-Assisted Solvent-Free Acetylation of Cellulose with Acetic Anhydride in the Presence of Iodine as a Catalyst. Molecules 2009, 14, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Song, J.; Huang, Q. Alternative Reaction Mechanism for the Cross-Linking of Gelatin with Glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.G.; Lewis, D.M.; Tapley, K.N. Characterization of Cellulose Aldehyde Using Fourier Transform Infrared Spectroscopy. J. Appl. Polym. Sci. 2001, 82, 1195–1202. [Google Scholar] [CrossRef]
- Salmon, S. The Influence of Physical Form and Annealing Conditions on Dye Sorption by Chitosan. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 1995. [Google Scholar]
- Shen, J.; Nada, A.A.; Abou-Zeid, N.Y.; Hudson, S.M. Synthesis of Chitosan Iodoacetamides via Carbodiimide Coupling Reaction: Effect of Degree of Substitution on the Hemostatic Properties. Carbohydr. Polym. 2020, 229, 115522. [Google Scholar] [CrossRef]
- Liu, X.; Nishi, N.; Tokura, S.; Sakairi, N. Chitosan Coated Cotton Fiber: Preparation and Physical Properties. Carbohydr. Polym. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In Vitro Enzymatic Digestibility of Glutaraldehyde-Crosslinked Chitosan Nanoparticles in Lysozyme Solution and Their Applicability in Pulmonary Drug Delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in Aqueous Solution, Reaction with Proteins, and Application to Enzyme Crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.A. Glutaraldehyde Cross-Linking. In Biocatalyst Design for Stability and Specificity; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 516, pp. 23–283. ISBN 9780841225183. [Google Scholar]
- Wakelyn, P.J. Cotton Fiber Chemistry and Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429141263. [Google Scholar]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand Binding. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef] [PubMed]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters Necessary to Define an Immobilized Enzyme Preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Fourati, M.; Roig, V.; Raynal, L. Experimental Study of Liquid Spreading in Structured Packings. Chem. Eng. Sci. 2012, 80, 1–15. [Google Scholar] [CrossRef]
- Wojtasik, J.; Gładyszewski, K.; Skiborowski, M.; Górak, A.; Piątkowski, M. Enzyme-Enhanced CO2 Absorption Process in Rotating Packed Bed. Chem. Pap. 2019, 73, 861–869. [Google Scholar] [CrossRef]
- Lebling, K.; Leslie-Bole, H.; Psarras, P.; Bridgwater, E.; Byrum, Z.; Pilorgé, H. Direct Air Capture: Assessing Impacts to Enable Responsible Scaling. World Resour. Inst. 2022, 1–28. [Google Scholar] [CrossRef]
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 18 July 2022).
- Faiz, R.; Al-Marzouqi, M. Mathematical Modeling for the Simultaneous Absorption of CO2 and H2S Using MEA in Hollow Fiber Membrane Contactors. J. Memb. Sci. 2009, 342, 269–278. [Google Scholar] [CrossRef]












| Sample ID | Wetting Time -Top (s) | Wetting Time -Bottom (s) | Spreading Speed -Top (mm/s) | Spreading Speed -Bottom (mm/s) |
|---|---|---|---|---|
| Neat cheesecloth | 3.40 | 3.37 | 2.85 | 2.90 |
| Chitosan coated | 44.98 | 7.25 | 0.14 | 0.69 |
| Chitosan coated with entrapped enzyme | 19.14 | 8.55 | 0.48 | 0.76 |
| Chitosan coated + surface covalent 3-D enzyme aggregate | 2.95 | 3.04 | 7.04 | 6.98 |
| Immobilization Preparation ID | Active Enzyme Loading (U/g of Cellulose) | CO2 Absorption (%) |
|---|---|---|
| Chitosan coated + surface covalent mono-layer | 0.014 | 71.2 |
| Chitosan coated + surface covalent 3-D aggregate | 0.188 | 66.7 |
| Chitosan entrapping NZCA + cross-linked | 0.212 | 56.5 |
| Chitosan entrapping NZCA + surface covalent mono-layer | 0.280 | n.a. 2 |
| Chitosan entrapping NZCA + surface covalent 3-D aggregate | 0.316 | 48.6 |
| Textile control (no enzyme) | 0 | 23.4 |
| Textile control + dissolved NZCA (7 U/L of solvent) 1 | 0 | 84.5 |
| Textile control + dissolved NZCA (26 U/L of solvent) 1 | 0 | 94.5 |
| Solvent Flow Rate (mL/min) | N2 Flow Rate (LPM) | CO2 Flow Rate (LPM) | Starting CO2% | Lowest CO2% | %CO2 Captured | Effect of Gas Flow Increase | Effect of Solvent Flow Decrease | Liquid to Gas Ratio (mL/L) |
|---|---|---|---|---|---|---|---|---|
| 120 | 3.6 | 0.4 | 10.9 | 3.4 | 68.8% | 30 | ||
| 120 | 7.2 | 0.8 | 11.1 | 6.3 | 43.2% | 62.9% | 15 | |
| 72 | 7.2 | 0.8 | 11 | 6.8 | 38.2% | 58.1% | 88.3% | 9 |
| 72 | 3.6 | 0.4 | 10.8 | 3.7 | 65.7% | 95.5% | 18 |
| Packing ID | 10% K2CO3 pH 10.51 | 20% K2CO3 pH 10.51 | 10% DMG pH 10.90 | 5% K2CO3 pH 10.50 | 5% DMG pH 10.76 | 5% MDEA pH 11.15 | 10% K2CO3 pH 10.47 | 30% K2CO3 pH 10.60 |
|---|---|---|---|---|---|---|---|---|
| Conventional Raschig ring packing | 3.6% | 4.5% | 5.4% | |||||
| No enzyme textile control packing | 23.4% | 20.9% | 23.6% | 31.3% | 22.0% | 24.3% | ||
| Surface-only covalent 3-D aggregate packing | 68.8% | 70.9% | 56.3% | 66.4% | 62.7% | 68.2% | 49.1% |
| Packing ID | Gas Flow (LPM) | Solvent Flow (mL/min) | L/G (mL/L) | Starting CO2% | Lowest CO2% | CO2 Capture Efficiency (%) |
|---|---|---|---|---|---|---|
| No-enzyme control | 4 | 120 | 30 | 5.8 | 4.4 | 24.1% |
| Enzyme packing | 4 | 120 | 30 | 5.8 | 1.8 | 69.0% |
| No-enzyme control | 4 | 120 | 30 | 26.2 | 21.6 | 17.6% |
| Enzyme packing | 4 | 120 | 30 | 26.1 | 13.3 | 49.0% |
| No-enzyme control | 1.6 | 120 | 75 | 24.6 | 14.9 | 39.4% |
| Enzyme packing | 1.6 | 120 | 75 | 24.7 | 3.0 | 87.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Yuan, Y.; Salmon, S. Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture. Catalysts 2022, 12, 1108. https://doi.org/10.3390/catal12101108
Shen J, Yuan Y, Salmon S. Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture. Catalysts. 2022; 12(10):1108. https://doi.org/10.3390/catal12101108
Chicago/Turabian StyleShen, Jialong, Yue Yuan, and Sonja Salmon. 2022. "Durable and Versatile Immobilized Carbonic Anhydrase on Textile Structured Packing for CO2 Capture" Catalysts 12, no. 10: 1108. https://doi.org/10.3390/catal12101108