Gold(I)-Catalyzed Tandem Synthesis of Polycyclic Dihydroquinazolinones
Abstract
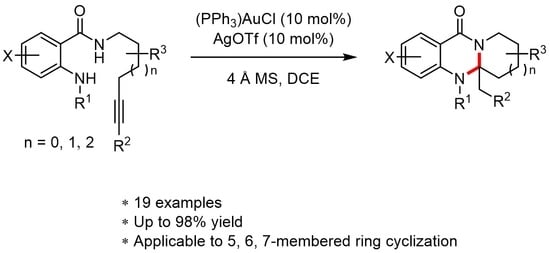
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Representative Procedure for the Synthesis of Dihydroquinazolinone 2a–u
3.2.1. 3a-Methyl-2,3,3a,4-tetrahydropyrrolo[2,1-b]quinazolin-9(1H)-one (2a)
3.2.2. 2,2,3a-Trimethyl-2,3,3a,4-tetrahydropyrrolo[2,1-b]quinazolin-9(1H)-one (2b)
3.2.3. 3a′-Methyl-3a′,4′-dihydro-1′H-spiro[cyclohexane-1,2′-pyrrolo[2,1-b]quinazolin]-9′(3′H)-one (2c)
3.2.4. 4b-Methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2d)
3.2.5. 3a-Ethyl-2,2-dimethyl-2,3,3a,4-tetrahydropyrrolo[2,1-b]quinazolin-9(1H)-one (2e)
3.2.6. 5a-Phenyl-5,5a,6,7,8,9-hexahydro-11H-pyrido[2,1-b]quinazolin-11-one (2f)
3.2.7. 5a-Methyl-5,5a,6,7,8,9-hexahydro-11H-pyrido[2,1-b]quinazolin-11-one (2g)
3.2.8. 2-methoxy-5a-methyl-5,5a,6,7,8,9-hexahydro-11H-pyrido[2,1-b]quinazolin-11-one (2h)
3.2.9. 2,3-dimethoxy-5a-methyl-5,5a,6,7,8,9-hexahydro-11H-pyrido[2,1-b]quinazolin-11-one (2i)
3.2.10. 5a,8,8-Trimethyl-5,5a,6,7,8,9-hexahydro-11H-pyrido[2,1-b]quinazolin-11-one (2j)
3.2.11. 13a-Methyl-5,6,13,13a-tetrahydro-8H-isoquinolino[1,2-b]quinazolin-8-one (2k)
3.2.12. 5a,9,9-Trimethyl-5a,6,7,8,9,10-hexahydroazepino[2,1-b]quinazolin-12(5H)-one (2l)
3.2.13. 8-Chloro-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2m)
3.2.14. 7-Chloro-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2n)
3.2.15. 8-Bromo-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2o)
3.2.16. 4b-methyl-7-nitro-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2p)
3.2.17. 4b,8-Dimethyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2q)
3.2.18. 8-Methoxy-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2r)
3.2.19. 7,8-dimethoxy-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2s)
3.2.20. 6,7,8-trimethoxy-4b-methyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2t)
3.2.21. 4b,5-dimethyl-4b,12-dihydroisoindolo[1,2-b]quinazolin-10(5H)-one (2u)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michael, J.P. Indolizidine and quinolizidine alkaloid. Nat. Prod. Rep. 2002, 19, 719–741. [Google Scholar] [CrossRef]
- Yamamoto, Y. Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. [Google Scholar] [CrossRef]
- Deiters, A.; Martin, S.F. Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis. Chem. Rev. 2004, 104, 2199–2238. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef] [PubMed]
- Rohokale, R.; Kshirsagar, U.A. Advanced synthetic strategies for constructing quinazolinone scaffolds. Synthesis 2016, 1253–1268. [Google Scholar]
- Badolato, M.; Aiello, F.; Neamati, N. 2,3-Dihydroquinazolin-4(1H)-one as a privileged scaffold in drug design. RSC Adv. 2018, 8, 20894–20921. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, K.; Madhumitha, G. Synthetic strategy with representation on mechanistic pathway for the therapeutic applications of dihydroquinazolinones. Eur. J. Med. Chem. 2016, 123, 596–630. [Google Scholar] [CrossRef]
- Li, S.; Ma, J.-A. Core-structure-inspired asymmetric addition reactions: Enantioselective synthesis of dihydrobenzoxazinone- and dihydroquinazolinone-based anti-HIV agents. Chem. Soc. Rev. 2015, 44, 7439–7448. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, S.D.; Argade, N.P. Aryne insertion reactions leading to bioactive fused quinazolinones: Diastereoselective total synthesis of Cruciferane. Org. Lett. 2013, 15, 4006–4009. [Google Scholar] [CrossRef]
- Jao, C.-W.; Lin, W.-C.; Wu, Y.-T.; Wu, P.-L. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from phaius mishmensis. J. Nat. Prod. 2018, 71, 1275–1279. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, L.; Song, J.; Li, X. Evodiamine: A review of its pharmacology, toxicity, pharmacokinetics and preparation researches. J. Ethnopharmacol. 2020, 262, 113164. [Google Scholar] [CrossRef]
- Wang, L.; Fang, K.; Cheng, J.; Li, Y.; Huang, Y.; Chen, S.; Dong, G.; Wu, S.; Sheng, C. Scaffold hopping of natural product evodiamine: Discovery of a novel antitumor scaffold with excellent potency against colon cancer. J. Med. Chem. 2020, 63, 696–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lai, Z.; Yuan, S.; Huang, Y.-Y.; Dong, G.; Sheng, C.; Ke, H.; Luo, H.-B. Discovery of evodiamine derivatives as highly selective PDE5 inhibitors targeting a unique allosteric pocket. J. Med. Chem. 2020, 63, 9828–9837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Ding, Q.; Li, X.; Fan, X.; Zhang, G. Alkylamino-directed one-pot reaction of N-alkyl anilines with CO, amines and aldehydes leading to 2,3-dihydroquinazolin-4(1H)-ones. Adv. Synth. Catal. 2019, 361, 976–982. [Google Scholar] [CrossRef]
- Cheng, X.; Vellalath, S.; Goddard, R.; List, B. Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes. J. Am. Chem. Soc. 2008, 130, 15786–15787. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, X.-L.; Ma, J.; Zhang, Y.-P.; Shi, Q.-C.; Luo, L.-J.; Song, B.-A.; Yang, S.; Hu, D.-Y. Preparation of 2,3-dihydroquinazolin-4(1H)-one derivatives in aqueous media with β-cyclodextrin-SO3H as a recyclable catalyst. Green Chem. 2014, 16, 3210–3217. [Google Scholar] [CrossRef]
- Chen, J.-X.; Su, W.-K.; Wu, H.-Y.; Liu, M.-C.; Jin, C. Eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-ones in ionic liquids or ionic liquid–water without additional catalyst. Green Chem. 2007, 9, 972–975. [Google Scholar] [CrossRef]
- Yoo, C.L.; Fettinger, J.C.; Kurth, M.J. Stannous chloride in alcohol: A one-pot conversion of 2-nitro-N-arylbenzamides to 2,3-dihydro-1H-quinazoline-4-ones. J. Org. Chem. 2005, 70, 6941–6943. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.-M.; Chen, H.; Shi, D.-Q. Efficient and convenient synthesis of spiroindolinone-quinazolines induced by stannous chloride. Tetrahedron 2011, 67, 9342–9346. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Montagnon, T.; Snyder, S.A. Tandem reactions, cascade sequences, and biomimetic strategies in total synthesis. Chem. Commun. 2003, 5, 551–564. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Malacria, M. Selective preparation of complex polycyclic molecules from acyclic precursors via radical mediated or transition metal-catalyzed cascade reactions. Chem. Rev. 1996, 96, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Wegner, H.A.; Auzias, M. Gold for C–C coupling reactions: A Swiss-Army-knife catalyst? Angew. Chem. Int. Ed. 2011, 50, 8236–8247. [Google Scholar] [CrossRef] [PubMed]
- Zi, W.; Toste, F.D. Recent advances in enantioselective gold catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589. [Google Scholar] [CrossRef]
- Ohno, H. Gold-catalyzed cascade reactions of alkynes for construction of polycyclic compounds. Isr. J. Chem. 2013, 53, 869–882. [Google Scholar] [CrossRef]
- Qian, D.; Zhang, J. Gold-catalyzed cascade reactions for synthesis of carbo- and heterocycles: Selectivity and diversity. Chem. Rec. 2014, 14, 280–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, Y.; Liu, H. Recent advances in the gold-catalyzed additions to C-C multiple bonds. Beilstein J. Org. Chem. 2011, 7, 897–936. [Google Scholar] [CrossRef] [Green Version]
- Barluenga, J.; Fernández, A.; Satrústegui, A.; Diéguez, A.; Rodríguez, F.; Fañanás, F.J. Tandem Intramolecular Hydroalkoxylation–Hydroarylation Reactions: Synthesis of enantiopure benzofused cyclic ethers from the chiral pool. Chem. Eur. J. 2008, 14, 4153–4156. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.K.; Bührle, M.; Wölfle, M.; Rudolph, M.; Wieteck, M.; Rominger, F.; Frey, W. Gold catalysis: Tandem reactions of diyne–diols and external nucleophiles as an easy access to tricyclic cage-like structures. Chem. Eur. J. 2010, 16, 9846–9854. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Mutyala, A.K.; Lakshmi, P.G.V.V.; Gajula, B.; Sridhar, B.; Pottireddygari, G.R.; Rao, T.P. Au(I)-catalyzed cascade reaction involving formal double hydroamination of alkynes bearing tethered carboxylic groups: An easy access to fused dihydrobenzimidazoles and tetrahydroquinazolines. J. Org. Chem. 2010, 75, 5963–5975. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-catalyzed tandem transformation: A simple approach for the synthesis of pyrrolo/pyrido[2, 1-a][1,3]benzoxazinones and pyrrolo/pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Lightstone, F.C.; Bruice, T.C. Enthalpy and entropy in ring closure reactions. Bioorg. Chem. 1998, 26, 193–199. [Google Scholar] [CrossRef]
- Casadei, M.A.; Galli, C.; Mandolini, L. Ring-closure reactions. 22. Kinetics of cyclization of diethyl (ω-bromoalkyl) malonates in the range of 4- to 21-membered rings. Role of ring strain. J. Am. Chem. Soc. 1984, 106, 1051–1056. [Google Scholar] [CrossRef]
- Galli, C.; Illuminati, G.; Mandolini, L.; Tamborra, P. Ring-closure reactions. 7. Kinetics and activation parameters of lactone formation in the range of 3- to 23-membered rings. J. Am. Chem. Soc. 1977, 99, 2591–2597. [Google Scholar] [CrossRef]
- Zhdanko, A.; Maier, M.E. Mechanistic Study of Gold(I)-catalyzed hydroamination of alkynes: Outer or inner sphere mechanism? Angew. Chem. Int. Ed. 2014, 53, 7760–7764. [Google Scholar] [CrossRef]
- Kovács, G.; Lledós, A.; Ujaque, G. Hydroamination of alkynes with ammonia: Unforeseen role of the gold(I) catalyst. Angew. Chem. Int. Ed. 2011, 50, 11147–11151. [Google Scholar] [CrossRef]





| Entry | Catalyst | Solvent | Time (h) | Yield (%) 2 | |
|---|---|---|---|---|---|
| 2a | 3a | ||||
| 1 | (PPh3)AuCl/AgOTf | toluene | 5 | 52 | 6 |
| 2 | (PPh3)AuCl/AgOTf | CH3CN | 24 | 15 | 10 |
| 3 | (PPh3)AuCl/AgOTf | THF | 2 | 67 | 5 |
| 4 | (PPh3)AuCl/AgOTf | CH2Cl2 | 4 | 68 | 2 |
| 5 | (PPh3)AuCl/AgOTf | dioxane | 1 | 76 | 8 |
| 6 | (PPh3)AuCl/AgOTf | CPME | 18 | 33 | 4 |
| 7 | (PPh3)AuCl/AgOTf | DMC | 4 | 66 | 0 |
| 8 | (PPh3)AuCl/AgOTf | DCE | 1 | 80 | 0 |
| 9 | (PPh3)AuCl/AgNO3 | DCE | 10 | 80 | 1 |
| 10 | (PPh3)AuCl/AgBF4 | DCE | 1 | 76 | 2 |
| 11 | (PPh3)AuCl/AgOTs | DCE | 1 | 74 | 1 |
| 12 | (PPh3)AuCl/AgSbF6 | DCE | 24 | 40 | 15 |
| 13 | (PPh3)AuCl | DCE | 24 | 17 | 1 |
| 14 | AgOTf | DCE | 24 | 23 | 0 |
| 15 | (PPh3)AuNTf2 | DCE | 2 | 78 | 7 |
| 16 | (XPhos)AuCl/AgOTf | DCE | 1 | 56 | 7 |
| 17 | (IPr)AuCl/AgOTf | DCE | 3 | 80 | 4 |
| 18 | (IPr)AuCl/AgNTf2 | DCE | 1 | 68 | 4 |
| 19 | (IPr)AuCl/AgNO3 | DCE | 24 | 20 | 4 |
| 20 3 | TfOH | DCE | 24 | 4 | 0 |
| 21 4 | (PPh3)AuCl/AgOTf | DCE | 1 | 76 | 0 |
| 22 5 | (PPh3)AuCl/AgOTf | DCE | 1 | 67 | 6 |

| Entry | Substrate | Product | T (°C ) | Time (h) | Yield (%) 2 |
|---|---|---|---|---|---|
| 1 |  |  | rt | 1.5 | 85 |
| 2 |  |  | rt | 3 | 90 |
| 3 |  |  | rt | 1 | 98 |
| 4 |  |  | 60 | 24 | 80 |
| 5 3 |  |  | 120 | 4 | 10 |
| 6 4 |  |  | 120 | 1 | 30 |
| 7 |  |  | 120 | 2.5 | 94 |
| 8 |  |  | 120 | 1.25 | 84 |
| 9 |  |  | 120 | 0.3 | 57 |
| 10 |  |  | 120 | 1 | 61 |
| 11 5 |  |  | 120 | 1 | 31 |
| 12 |  |  | rt | 0.5 | 96 |
| 13 |  |  | rt | 0.5 | 97 |
| 14 |  |  | rt | 2 | 92 |
| 15 |  |  | rt | 0.5 | 97 |
| 16 |  |  | rt | 8 | 91 |
| 17 |  |  | rt | 24 | 76 |
| 18 |  |  | rt | 3 | 72 |
| 19 |  |  | rt | 1 | 86 |
| 20 |  |  | 60 | 1.5 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Song, Y.; Ryu, J.-S. Gold(I)-Catalyzed Tandem Synthesis of Polycyclic Dihydroquinazolinones. Catalysts 2021, 11, 1436. https://doi.org/10.3390/catal11121436
Sun J, Song Y, Ryu J-S. Gold(I)-Catalyzed Tandem Synthesis of Polycyclic Dihydroquinazolinones. Catalysts. 2021; 11(12):1436. https://doi.org/10.3390/catal11121436
Chicago/Turabian StyleSun, Jingyang, Yoona Song, and Jae-Sang Ryu. 2021. "Gold(I)-Catalyzed Tandem Synthesis of Polycyclic Dihydroquinazolinones" Catalysts 11, no. 12: 1436. https://doi.org/10.3390/catal11121436