Atomic-Level Functionalized Graphdiyne for Electrocatalysis Applications
Abstract
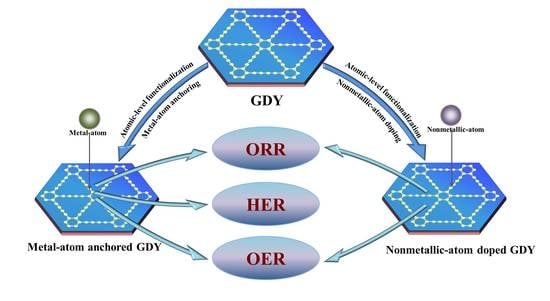
:1. Introduction
2. Synthesis of Atomic-Level Functionalized GDY Catalysts
2.1. Synthesis of Metal-Atom-Anchored GDY Catalysts
2.1.1. Wet Chemical Method
2.1.2. Electrochemical-Deposition Method
2.2. Synthesis of Nonmetallic-Atom-Doped GDY Catalysts
3. Theoretical Predictions
3.1. ORR
3.1.1. Metal-Atom-Anchored GDY Electrocatalysts
3.1.2. Nonmetallic-Atom-Doped GDY Electrocatalysts
3.2. HER
Metal-Atom-Anchored GDY Electrocatalysts
3.3. OER
Metal-Atom-Anchored GDY Electrocatalysts
4. Experimental Investigations
4.1. ORR
4.1.1. Metal-Atom-Anchored GDY Electrocatalysts
4.1.2. Nonmetallic-Atom-Doped GDY Electrocatalysts
4.2. HER
Metal-Atom-Anchored GDY Electrocatalysts
4.3. OER
4.3.1. Metal-Atom-Anchored GDY Electrocatalysts
4.3.2. Nonmetallic-Atom-Doped GDY Electrocatalysts
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rod, B.; Jeremy, M.; Bryan, P.; Seung, K.Y.; Rangachary, M.; Nancy, G.; Deborah, M.; Mahlon, W.; Fernando, G.; David, W.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Budischak, C.; Sewell, D.; Thomson, H.; Mach, L.; Veron, D.E.; Kempton, W. Cost-minimized combinations of wind power, solar power and electrochemical storage, powering the grid up to 99.9% of the time. J. Power Sources 2013, 225, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Wang, T.; Xie, H.; Chen, M.; D’Aloia, A.; Cho, J.; Wu, G.; Li, Q. Precious metal-free approach to hydrogen electrocatalysis for energy conversion: From mechanism understanding to catalyst design. Nano Energy 2017, 42, 69–89. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Lee, H. Three-dimensional bimetal TMO supported carbon based electrocatalyst developed via dry synthesis for hydrogen and oxygen evolution. Appl. Surf. Sci. 2020, 505. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Naohara, H.; Choi, Y.; Cai, Y.; Chen, W.F.; Liu, P.; Adzic, R.R. Highly stable Pt monolayer on PdAu nanoparticle electrocatalysts for the oxygen reduction reaction. Nat. Commun. 2012, 3, 1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M (Ni,Co,Fe,Mn) hydr (oxy) oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Sirringhaus, H.; Brown, P.; Friend, R.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.; Spiering, A.; Janssen, R.A.; Meijer, E.; Herwig, P. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Mortazavi, B.; Makaremi, M.; Shahrokhi, M.; Fan, Z.; Rabczuk, T. N-graphdiyne two-dimensional nanomaterials: Semiconductors with low thermal conductivity and high stretchability. Carbon 2018, 137, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Shang, H.; Zuo, Z.; Li, L.; Wang, F.; Liu, H.; Li, Y.; Li, Y. Ultrathin Graphdiyne Nanosheets Grown In Situ on Copper Nanowires and Their Performance as Lithium-Ion Battery Anodes. Angew. Chem. Int. Ed. 2018, 57, 774–778. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Tu, Z.; Zhao, F.; He, J.; Guan, Z.; Huang, C.; Yi, Y.; Li, Y. Synthesis and Electronic Structure of Boron-Graphdiyne with an sp-Hybridized Carbon Skeleton and Its Application in Sodium Storage. Angew. Chem. Int. Ed. 2018, 57, 3968–3973. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, X.; Wang, N.; He, J.; Li, X.; Wang, X.; Hou, Z.; Wang, K.; Gao, J.; Jiu, T.; et al. Graphdiyne Containing Atomically Precise N Atoms for Efficient Anchoring of Lithium Ion. ACS Appl. Mater. Interfaces 2019, 11, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-Containing Carbon Nitride Compounds: A New Functional Organic-Metal Hybrid Material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline Graphdiyne Nanosheets Produced at a Gas/Liquid or Liquid/Liquid Interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Shi, R.; Zhang, Y.; He, Z.; Gao, L.; Wang, R.; Shu, Y.; Ma, C.; Ge, Y.; et al. Graphdiyne as a Promising Mid-Infrared Nonlinear Optical Material for Ultrafast Photonics. Adv. Opt. Mater. 2020, 8, 2000067. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts 2018, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-S.; Li, Y.-L. Structure of 2D Graphdiyne and Its Application in Energy Fields. Acta Phys. Chim. Sin. 2016, 32, 1314–1329. [Google Scholar] [CrossRef]
- Xue, Z.; Zhu, M.; Dong, Y.; Feng, T.; Chen, Z.; Feng, Y.; Shan, Z.; Xu, J.; Meng, S. An integrated targeting drug delivery system based on the hybridization of graphdiyne and MOFs for visualized cancer therapy. Nanoscale 2019, 11, 11709–11718. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xue, Y.; Li, Y. Graphdiyne and its Assembly Architectures: Synthesis, Functionalization, and Applications. Adv. Mater. 2019, 31, 1803101. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, D.; Zhang, J.; Lu, F.; Li, Y. Synthesis and Applications of Graphdiyne-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1803762. [Google Scholar] [CrossRef]
- Qi, H.; Yu, P.; Wang, Y.; Han, G.; Liu, H.; Yi, Y.; Li, Y.; Mao, L. Graphdiyne Oxides as Excellent Substrate for Electroless Deposition of Pd Clusters with High Catalytic Activity. J. Am. Chem. Soc. 2015, 137, 5260–5263. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Li, J.; Liao, W.; Li, Z.; Zhang, J.; Chen, C. Pyrolysis-induced synthesis of iron and nitrogen-containing carbon nanolayers modified graphdiyne nanostructure as a promising core-shell electrocatalyst for oxygen reduction reaction. Carbon 2017, 119, 201–210. [Google Scholar] [CrossRef]
- Shi, G.; Yu, C.; Fan, Z.; Li, J.; Yuan, M. Graphdiyne-Supported NiFe Layered Double Hydroxide Nanosheets as Functional Electrocatalysts for Oxygen Evolution. ACS Appl. Mater. Interfaces 2019, 11, 2662–2669. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Yang, Q.; Ma, L.; Zhao, Y.; Huang, Z.; Li, X.; Dong, B.; Fu, X.Z.; Zhi, C. Metal-Tuned Acetylene Linkages in Hydrogen Substituted Graphdiyne Boosting the Electrochemical Oxygen Reduction. Small 2020, 16, 1907341. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef] [Green Version]
- Haley, M.M. Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures. Pure Appl. Chem. 2008, 80, 519–532. [Google Scholar] [CrossRef]
- He, J.; Ma, S.Y.; Zhou, P.; Zhang, C.X.; He, C.; Sun, L.Z. Magnetic Properties of Single Transition-Metal Atom Absorbed Graphdiyne and Graphyne Sheet from DFT+U Calculations. J. Phys. Chem. C 2012, 116, 26313–26321. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Li, Y. Emerging Electrochemical Energy Applications of Graphdiyne. Joule 2019, 3, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Li, Y.; Wang, N.; Xue, Y.; Zuo, Z.; Liu, H.; Li, Y. Progress in Research into 2D Graphdiyne-Based Materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, X.; Zhou, J.; Xu, H.; Li, Z.; Zhang, S.; Xie, Z.; Zhang, J.; Liu, Z. Chemical Vapor Deposition Growth of Linked Carbon Monolayers with Acetylenic Scaffoldings on Silver Foil. Adv. Mater. 2017, 29, 1604665. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Bao, W.; Shi, R.; Zhao, J.; Kang, J.; Xia, X.; Chen, H.; Li, H.; Xu, J.; et al. Graphdiyne Nanosheets for Multicolor Random Lasers. ACS Appl. Nano Mater. 2020. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of Graphdiyne Nanowalls Using Acetylenic Coupling Reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Qian, X.; Liu, H.; Lin, H.; Chen, N.; Li, Y. Construction of Tubular Molecule Aggregations of Graphdiyne for Highly Efficient Field Emission. J. Phys. Chem. C 2011, 115, 2611–2615. [Google Scholar] [CrossRef]
- Zuo, Z.; Shang, H.; Chen, Y.; Li, J.; Liu, H.; Li, Y.; Li, Y. A facile approach for graphdiyne preparation under atmosphere for an advanced battery anode. Chem. Commun. 2017, 53, 8074–8077. [Google Scholar] [CrossRef]
- Gao, X.; Ren, H.; Zhou, J.; Du, R.; Yin, C.; Liu, R.; Peng, H.; Tong, L.; Liu, Z.; Zhang, J. Synthesis of Hierarchical Graphdiyne-Based Architecture for Efficient Solar Steam Generation. Chem. Mater. 2017, 29, 5777–5781. [Google Scholar] [CrossRef]
- Cirera, B.; Zhang, Y.Q.; Bjork, J.; Klyatskaya, S.; Chen, Z.; Ruben, M.; Barth, J.V.; Klappenberger, F. Synthesis of extended graphdiyne wires by vicinal surface templating. Nano Lett. 2014, 14, 1891–1897. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Y.; Zhao, J.; Ma, D.; Huang, W.; Zhang, Y.; Wang, Y.; Jiang, X.; Xiang, Y.; Li, J.; et al. Kerr Nonlinearity in 2D Graphdiyne for Passive Photonic Diodes. Adv. Mater. 2019, 31, 1807981. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Semin, S.; Feng, Y.; Xu, J.; Rasing, T. Solvent induced enhancement of nonlinear optical response of graphdiyne. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and Properties of 2D Carbon—Graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Hernandez, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Sciences 2016, 353, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.M. Catalysis: Tens of thousands of atoms replaced by one. Nature 2015, 525, 325–326. [Google Scholar] [CrossRef]
- Lin, Z.-Z. Graphdiyne as a promising substrate for stabilizing Pt nanoparticle catalyst. Carbon 2015, 86, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.W.; Li, T.; Wang, Q.; Yang, G.; He, C.; Ma, B.; Lu, Z. Graphyne as a promising substrate for the noble-metal single-atom catalysts. Carbon 2015, 95, 756–765. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Jiang, X.; Li, X.-B.; Liu, Z.; Zhang, J.; Tung, C.-H.; Wu, L.-Z. Graphdiyne: A Promising Catalyst–Support To Stabilize Cobalt Nanoparticles for Oxygen Evolution. ACS Catal. 2017, 7, 5209–5213. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Vahedi, A.M.; Ardjmand, M.; Ahangari, M.G. Investigation of heavy metal atoms adsorption onto graphene and graphdiyne surface: A density functional theory study. Superlattices Microstruct. 2016, 100, 1094–1102. [Google Scholar] [CrossRef]
- Lin, J.; Qiao, B.; Li, N.; Li, L.; Sun, X.; Liu, J.; Wang, X.; Zhang, T. Little do more: A highly effective Pt(1)/FeO(x) single-atom catalyst for the reduction of NO by H2. Chem. Commun. 2015, 51, 7911–7914. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.P.; Wang, H.J.; Tang, S.F.; Lu, X.L.; Shu, M.; Si, R.; Lu, T.B. Engineering the Coordination Environment of Single-Atom Platinum Anchored on Graphdiyne for Optimizing Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 9382–9386. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Xue, Y.; Yu, H.; Liu, Y.; Fang, Y.; Xing, C.; Huang, B.; Li, Y. Highly Efficient and Selective Generation of Ammonia and Hydrogen on a Graphdiyne-Based Catalyst. J. Am. Chem. Soc. 2019, 141, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hui, L.; Xue, Y.; Liu, Y.; Fang, Y.; Xing, C.; Zhang, C.; Zhang, D.; Chen, X.; Du, Y.; et al. 2D graphdiyne loading ruthenium atoms for high efficiency water splitting. Nano Energy 2020, 72, 104667. [Google Scholar] [CrossRef]
- McKeown, D.A.; Hagans, P.L.; Carette, L.P.; Russell, A.E.; Swider, K.E.; Rolison, D.R. Structure of hydrous ruthenium oxides: Implications for charge storage. J. Phys. Chem. B 1999, 103, 4825–4832. [Google Scholar] [CrossRef]
- Ribeiro, J.; Tremiliosi-Filho, G.; Olivi, P.; de Andrade, A.R. XAS characterization of the RuO2–Ta2O5 system local (crystal) structure. Mater. Chem. Phys. 2011, 125, 449–460. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, B.; Yi, Y.; Guo, Y.; Zuo, Z.; Li, Y.; Jia, Z.; Liu, H.; Li, Y. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat. Commun. 2018, 9, 1460. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Huang, B.; Hui, L.; Zhang, C.; Fang, Y.; Liu, Y.; Zhao, Y.; Li, Y.; Liu, H.; et al. Ultrathin Nanosheet of Graphdiyne-Supported Palladium Atom Catalyst for Efficient Hydrogen Production. IScience 2019, 11, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Li, Y.; Zhang, J.; Liu, Z.; Zhao, Y. 2D graphdiyne materials: Challenges and opportunities in energy field. Sci. China Chem. 2018, 61, 765–786. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Liu, N.; Liu, M.; Wang, Y.; Liu, Z. Synthesis of nitrogen-doped graphene using embedded carbon and nitrogen sources. Adv. Mater. 2011, 23, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.-X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-Doped Graphene via Solvothermal Synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Ghosh, A.; Late, D.J.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. NO2 and humidity sensing characteristics of few-layer graphenes. J. Exp. Nanosci. 2009, 4, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Chipeng, X.; Ning, W.; Xiaofang, L.; Guorong, X.; Changshui, H. Research on the Preparation of Graphdiyne and Its Derivatives. Chemistry 2020, 26, 569–583. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, W.; Wang, K.; Song, Y.; Zhao, F.; Wang, N.; Long, Y.; Wang, H.; Huang, C. Chemical Modification of the sp-Hybridized Carbon Atoms of Graphdiyne by Using Organic Sulfur. Chemistry 2019, 25, 5643–5647. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Yao, H.; Liu, D.; Song, L.; Zhu, J.; Li, S.; Gu, L.; Lin, K.; Wang, D. Stereodefined Codoping of sp-N and S Atoms in Few-Layer Graphdiyne for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 7240–7244. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Y.; He, H.; Zhang, Y.; Liu, R.; Cao, H.; Wang, M.; Liu, J.; Zhang, G.; Li, Y.; et al. Heteroatom doped graphdiyne as efficient metal-free electrocatalyst for oxygen reduction reaction in alkaline medium. J. Mater. Chem. A 2016, 4, 4738–4744. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Rong, W.; Long, B.; Ji, Y.; Duan, L. Corrosion-Induced Cl-Doped Ultrathin Graphdiyne toward Electrocatalytic Nitrogen Reduction at Ambient Conditions. ACS Catal. 2019, 9, 10649–10655. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, N.; Si, W.; Hou, Z.; Li, X.; Wang, X.; Zhao, F.; Yang, Z.; Zhang, Y.; Huang, C. Pyridinic nitrogen exclusively doped carbon materials as efficient oxygen reduction electrocatalysts for Zn-air batteries. Appl. Catal. B 2020, 261, 118234. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, J.; Yao, H.; Zhang, L.; Lin, K.; Wang, L.; Yang, N.; Liu, D.; Song, L.; Zhu, J.; et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis. Nat. Chem. 2018, 10, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, H.; Li, Y.; Yi, Y.; Shang, X.; Zhang, S.; Yu, X.; Zhang, S.; Cao, H.; Zhang, G. Nitrogen-doped graphdiyne as a metal-free catalyst for high-performance oxygen reduction reactions. Nanoscale 2014, 6, 11336–11343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowicki, P.; Pietrzak, R.; Wachowska, H. X-ray Photoelectron Spectroscopy Study of Nitrogen-Enriched Active Carbons Obtained by Ammoxidation and Chemical Activation of Brown and Bituminous Coals. Energy Fuels 2010, 24, 1197–1206. [Google Scholar] [CrossRef]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Ihm, K.; Kang, T.-H.; Lee, D.H.; Park, S.-Y.; Kim, K.-J.; Kim, B.; Yang, J.H.; Park, C.Y. Oxygen contaminants affecting on the electronic structures of the carbon nano tubes grown by rapid thermal chemical vapor deposition. Surf. Sci. 2006, 600, 3729–3733. [Google Scholar] [CrossRef]
- Xue, Q.; Ding, Y.; Xue, Y.; Li, F.; Chen, P.; Chen, Y. 3D nitrogen-doped graphene aerogels as efficient electrocatalyst for the oxygen reduction reaction. Carbon 2018, 139, 137–144. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jimenez, M.J.; Ye, G.; Dong Kim, N.; Samuel, E.L.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, N.; Wang, Z.; Zhao, J. Nitrogen-Doped Graphene on Transition Metal Substrates as Efficient Bifunctional Catalysts for Oxygen Reduction and Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2017, 9, 22578–22587. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Recent Advances in Atomic Metal Doping of Carbon-based Nanomaterials for Energy Conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Du, P.; Zhang, H.; Cai, C. Graphdiyne as a metal-free catalyst for low-temperature CO oxidation. Phys. Chem. Chem. Phys. 2014, 16, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Lv, P.; He, C.; Ma, D.; Yang, Z. First principles study on the interfacial properties of NM/graphdiyne (NM = Pd, Pt, Rh and Ir): The implications for NM growing. Appl. Surf. Sci. 2016, 360, 1–7. [Google Scholar] [CrossRef]
- Sun, M.; Wu, T.; Xue, Y.; Dougherty, A.W.; Huang, B.; Li, Y.; Yan, C.-H. Mapping of atomic catalyst on graphdiyne. Nano Energy 2019, 62, 754–763. [Google Scholar] [CrossRef]
- Feng, Z.; Li, R.; Ma, Y.; Li, Y.; Wei, D.; Tang, Y.; Dai, X. Molecule-level graphdiyne coordinated transition metals as a new class of bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions. Phys. Chem. Chem. Phys. 2019, 21, 19651–19659. [Google Scholar] [CrossRef]
- NøRskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Fu, Z.; Yang, Z. Strong metal-support interactions impart activity in the oxygen reduction reaction: Au monolayer on Mo2C (MXene). J. Phys. Condens. Matter 2018, 30, 475201. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Meng, C.; Han, Y. Palladium Nanoparticles/Defective Graphene Composites as Oxygen Reduction Electrocatalysts: A First-Principles Study. J. Phys. Chem. C 2012, 116, 2710–2719. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; Stevenson, K.J. Influence of Nitrogen Doping on Oxygen Reduction Electrocatalysis at Carbon Nanofiber Electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ma, Y.; Li, Y.; Li, R.; Liu, J.; Li, H.; Tang, Y.; Dai, X. Graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites as potential metal-free electrocatalysts for oxygen reduction reaction. J. Phys. Condens. Matter 2019, 31, 465201. [Google Scholar] [CrossRef]
- Feng, Z.; Ma, Y.; Li, Y.; Li, R.; Tang, Y.; Dai, X. Charge-compensated co-doping of graphdiyne with boron and nitrogen to form metal-free electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2020, 22, 1493–1501. [Google Scholar] [CrossRef]
- Kang, B.; Wu, S.; Ma, J.; Ai, H.; Lee, J.Y. Synergy of sp-N and sp 2-N codoping endows graphdiyne with comparable oxygen reduction reaction performance to Pt. Nanoscale 2019, 11, 16599–16605. [Google Scholar] [CrossRef]
- Gu, J.; Magagula, S.; Zhao, J.; Chen, Z. Boosting ORR/OER Activity of Graphdiyne by Simple Heteroatom Doping. Small Methods 2019, 3, 1800550. [Google Scholar] [CrossRef]
- Chen, X.; Ong, W.-J.; Kong, Z.; Zhao, X.; Li, N. Probing the active sites of site-specific nitrogen doping in metal-free graphdiyne for electrochemical oxygen reduction reactions. Sci. Bull. 2020, 65, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Das, B.K.; Sen, D.; Chattopadhyay, K.K. Implications of boron doping on electrocatalytic activities of graphyne and graphdiyne families: A first principles study. Phys. Chem. Chem. Phys. 2016, 18, 2949–2958. [Google Scholar] [CrossRef]
- Ketabi, N.; Tolhurst, T.M.; Leedahl, B.; Liu, H.; Li, Y.; Moewes, A. How functional groups change the electronic structure of graphdiyne: Theory and experiment. Carbon 2017, 123, 1–6. [Google Scholar] [CrossRef]
- Das, B.K.; Sen, D.; Chattopadhyay, K.K. Nitrogen doping in acetylene bonded two dimensional carbon crystals: Ab-initio forecast of electrocatalytic activities vis-à-vis boron doping. Carbon 2016, 105, 330–339. [Google Scholar] [CrossRef]
- Ren, H.; Shao, H.; Zhang, L.; Guo, D.; Jin, Q.; Yu, R.; Wang, L.; Li, Y.; Wang, Y.; Zhao, H.; et al. A New Graphdiyne Nanosheet/Pt Nanoparticle-Based Counter Electrode Material with Enhanced Catalytic Activity for Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5, 1500296. [Google Scholar] [CrossRef]
- Kondori, A.; Esmaeilirad, M.; Baskin, A.; Song, B.; Wei, J.; Chen, W.; Segre, C.U.; Shahbazian-Yassar, R.; Prendergast, D.; Asadi, M. Identifying Catalytic Active Sites of Trimolybdenum Phosphide (Mo3P) for Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2019, 9, 1900516. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.J.; Ito, Y.; Cong, W.; Tan, Y.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. Int. Ed. 2015, 54, 14031–14035. [Google Scholar] [CrossRef]
- Sun, M.; Dougherty, A.W.; Huang, B.; Li, Y.; Yan, C.H. Accelerating Atomic Catalyst Discovery by Theoretical Calculations-Machine Learning Strategy. Adv. Energy Mater. 2020, 10, 1903949. [Google Scholar] [CrossRef]
- Guo, J.; Shi, R.; Wang, R.; Wang, Y.; Zhang, F.; Wang, C.; Chen, H.; Ma, C.; Wang, Z.; Ge, Y.; et al. Graphdiyne-Polymer Nanocomposite as a Broadband and Robust Saturable Absorber for Ultrafast Photonics. Laser Photonics Rev. 2020, 14, 1900367. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, P.; Guo, J.; Shi, R.; Huang, W.; Shi, Z.; Wu, L.; Zhang, F.; Gao, L.; Li, C.; et al. Graphdiyne-Based Flexible Photodetectors with High Responsivity and Detectivity. Adv. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jiang, P.H.; Liu, H.J.; Fan, D.D.; Liang, J.H.; Wei, J.; Cheng, L.; Zhang, J.; Shi, J. Graphdiyne: A two-dimensional thermoelectric material with high figure of merit. Carbon 2015, 90, 255–259. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Matta, S.K.; Will, G.; Du, A. Transition-Metal Single Atoms Anchored on Graphdiyne as High-Efficiency Electrocatalysts for Water Splitting and Oxygen Reduction. Small Methods 2019, 3, 1800419. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S.; Guo, S.; Su, D.; Sun, S. Synthetic control of FePtM nanorods (M = Cu, Ni) to enhance the oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 7130–7133. [Google Scholar] [CrossRef]
- Li, H.-H.; Ma, S.-Y.; Fu, Q.-Q.; Liu, X.-J.; Wu, L.; Yu, S.-H. Scalable Bromide-Triggered Synthesis of Pd@Pt Core–Shell Ultrathin Nanowires with Enhanced Electrocatalytic Performance toward Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 7862–7868. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Dong, Y.; Hao, F.; Chen, L. Recent progress in single-atom electrocatalysts: Concept, synthesis, and applications in clean energy conversion. J. Mater. Chem. A 2018, 6, 14025–14042. [Google Scholar] [CrossRef]
- Alarawi, A.; Ramalingam, V.; He, J.-H. Recent advances in emerging single atom confined two-dimensional materials for water splitting applications. Mater. Today Energy 2019, 11, 1–23. [Google Scholar] [CrossRef]
- Chen, Z.W.; Chen, L.X.; Yang, C.C.; Jiang, Q. Atomic (single, double, and triple atoms) catalysis: Frontiers, opportunities, and challenges. J. Mater. Chem. A 2019, 7, 3492–3515. [Google Scholar] [CrossRef]
- Xiao-Feng, Y.; Aiqin, W.; Botao, Q.; Jun, L.; Jingyue, L.; Tao, Z. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Karuppannan, M.; Vinothkannan, M.; Ramachandran, K.; Kwon, O.J.; Yoo, D.J. Ultrafine Pt Nanoparticles Stabilized by MoS2/N-Doped Reduced Graphene Oxide as a Durable Electrocatalyst for Alcohol Oxidation and Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2019, 11, 12504–12515. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, Z.; Wu, X.; Lv, Z.; Wu, P.; Cai, C. Graphdiyne-Supported Single-Atom-Sized Fe Catalysts for the Oxygen Reduction Reaction: DFT Predictions and Experimental Validations. ACS Catal. 2018, 8, 10364–10374. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Yang, W.-H.; Wang, H.-H.; Chen, C.; Zhou, Z.-Y.; Sun, S.-G. Pyrolyzed Fe–N–C Composite as an Efficient Non-precious Metal Catalyst for Oxygen Reduction Reaction in Acidic Medium. ACS Catal. 2014, 4, 3928–3936. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Shi, G.; Xie, Y.; Du, L.; Fan, Z.; Chen, X.; Fu, X.; Xie, W.; Wang, M.; Yuan, M. Stabilization of cobalt clusters with graphdiyne enabling efficient overall water splitting. Nano Energy 2020, 74, 104852. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Mullen, K.; Feng, X. Carbon-Rich Nanomaterials: Fascinating Hydrogen and Oxygen Electrocatalysts. Adv. Mater. 2018, 30, 1800528. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zheng, L.; Shi, X.; Zheng, H. Carbon nanomaterials with sp or/and sp hybridization in energy conversion and storage applications: A review. Energy Storage Mater. 2020, 26, 349–370. [Google Scholar] [CrossRef]


















| Catalysts 1 | Synthesis Method | Eonset (V vs. RHE) | E1/2 (V vs. RHE) | Id (mA cm−2) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|---|
| Fe/GDY | chemical reduction | 0.21 | 0.1 | 6.7 (0.1 V) | 63 | [135] |
| NGDY | high-temperature annealing | N/A | 0.87 | 38.0 (0.75 V) | 60 | [82] |
| NFGDY | high-temperature annealing | 1.0 | 0.74 | 4.5 (0 V) | N/A | [79] |
| Catalysts | Synthesis Method | η At 10 mA cm−2 (mV) | Eonset (V vs. RHE) | j0 (mA cm−2) | Tafel Slope (mV dec−1) | TOF (s−1) | Mass (A mgmetal−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ni/GDY 1 | electrochemical-deposition | 86 | 23 | 0.25 (0 V) | 45.8 | 1.59 (0.1 V) | 16.6 (0.2 V) | [67] |
| Fe/GDY 1 | electrochemical-deposition | 66 | 9 | 0.29 (0 V) | 37.8 | 4.15 (0.1 V) | 80.0 (0.2 V) | [67] |
| Pd0/GDY 1 | electrochemical-deposition | 55 | 11 | 0.28 (0 V) | 47 | 16.7 (0.1 V) | 61.5 (0.2 V) | [68] |
| Ru/GDY 1 | in situ reduction | 44 | N/A | 0.70 (0 V) | 30 | 8.45 (−0.1 V) | 15.88 (0.15 V) | [64] |
| Cu@GDY-Co 2 | absorption–reduction | 63 | N/A | N/A | 41.7 | N/A | N/A | [139] |
| Catalysts 1 | Synthesis Method | η At 10 mA cm−2 (mV) | j0 (mA cm−2) | Tafel Slope (mV dec−1) | TOF (s−1) | Mass (A mgmetal−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Ru/GDY 1 | in situ reduction | 531 | 0.084 (0 V) | 100 | 7.09 (2.0 V) | 9.03 (2.0 V) | [64] |
| Cu@GDY-Co 2 | absorption–reduction | 234 | N/A | 51.7 | N/A | N/A | [139] |
| NSFLGDY-900 2 | high-temperature annealing | 299 | 47.2 (1.6 V) | 62 | N/A | N/A | [78] |
| NSFLGDY-900a 2 | high-temperature annealing | 308 | 35.7 (1.6 V) | 66 | N/A | N/A | [78] |
| NSFLGDY-900b 2 | high-temperature annealing | N/A | 4.6 (1.6 V) | 79 | N/A | N/A | [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Zheng, Y.; Chen, S.; Xu, J. Atomic-Level Functionalized Graphdiyne for Electrocatalysis Applications. Catalysts 2020, 10, 929. https://doi.org/10.3390/catal10080929
Qian X, Zheng Y, Chen S, Xu J. Atomic-Level Functionalized Graphdiyne for Electrocatalysis Applications. Catalysts. 2020; 10(8):929. https://doi.org/10.3390/catal10080929
Chicago/Turabian StyleQian, Xiaodong, Yongshen Zheng, Songhua Chen, and Jialiang Xu. 2020. "Atomic-Level Functionalized Graphdiyne for Electrocatalysis Applications" Catalysts 10, no. 8: 929. https://doi.org/10.3390/catal10080929