Arsenic Removal by Advanced Electrocoagulation Processes: The Role of Oxidants Generated and Kinetic Modeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Benchmarking Conventional Electrocoagulation with Advanced Technologies
2.2. Arsenic Speciation during Electrochemical Treatment
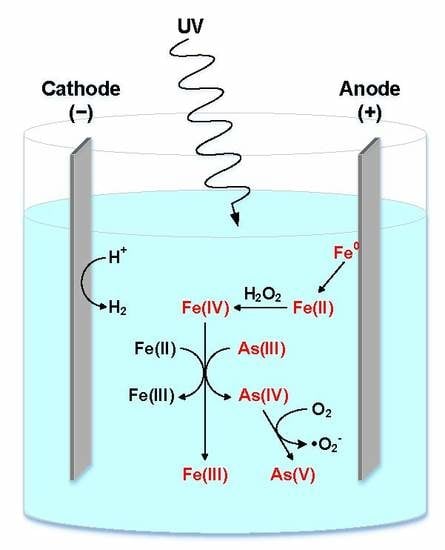
2.3. Understanding the Role of Oxidants in Arsenic Removal
2.4. Understanding the Mechanism of Arsenic Oxidation in Advanced Oxidation Processes
3. Materials and Methods
3.1. Reagents
3.2. Experimental Setup and Procedure
3.3. Analytical Methods and Instruments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution; Wiley-Blackwell: Oxford, UK, 2009; ISBN 9781444308785. [Google Scholar]
- Brammer, H.; Ravenscroft, P. Arsenic in groundwater: A threat to sustainable agriculture in South and South-east Asia. Environ. Int. 2009, 35, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Sy, S.M.T.; Salud-Gnilo, C.M.; Yap-Silva, C.; Tababa, E.J.L. A retrospective review of the dermatologic manifestations of chronic arsenic poisoning in the Philippines. Int. J. Dermatol. 2017, 56, 721–725. [Google Scholar] [CrossRef]
- Kleinendorst, T.; Petrusevski, B.; Ramos, E.; Muijtjens, R. DRR Team Central Luzon Mission Report; Dutch Risk Reduction Team: Dutch, The Netherlands, 2015; Available online: http://www.drrteam-dsswater.nl/wp-content/uploads/2015/06/DRR-Central-Luzon-mission-report-V5-final.pdf (accessed on 10 April 2018).
- Drahota, P.; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Ratna Kumar, P.; Chaudhari, S.; Khilar, K.C.; Mahajan, S. Removal of arsenic from water by electrocoagulation. Chemosphere 2004, 55, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human Health Effects From Chronic Arsenic Poisoning–A Review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Casentini, B.; Rossetti, S.; Fazi, S.; Puig, S.; Aulenta, F. Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: A novel approach to the bioremediation of arsenic-polluted groundwater. J. Hazard. Mater. 2015, 283, 617–622. [Google Scholar] [CrossRef]
- Dodd, M.C.; Vu, N.D.; Ammann, A.; Le, V.C.; Kissner, R.; Pham, H.V.; Cao, T.H.; Berg, M.; von Gunten, U. Kinetics and Mechanistic Aspects of As(III) Oxidation by Aqueous Chlorine, Chloramines, and Ozone: Relevance to Drinking Water Treatment. Environ. Sci. Technol. 2006, 40, 3285–3292. [Google Scholar] [CrossRef]
- Li, N.; Fan, M.; Van Leeuwen, J.; Saha, B.; Yang, H.; Huang, C.P. Oxidation of As(III) by potassium permanganate. J. Environ. Sci. 2007, 19, 783–786. [Google Scholar] [CrossRef]
- Lazarova, Z.; Sorlini, S.; Prandini, F.; Staniloae, D. Arsenic removal processes. In Best Practice Guide on Metals Removal from Drinking Water by Treatment; IWA Publishing: London, UK, 2012; pp. 83–94. [Google Scholar]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Yoon, S.-H.H.; Lee, J.H.; Oh, S.; Yang, J.E. Photochemical oxidation of As(III) by vacuum-UV lamp irradiation. Water Res. 2008, 42, 3455–3463. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Van Genuchten, C.M.; Addy, S.E.A.; Yao, J.; Gao, N.; Gadgil, A.J. Modeling As(III) oxidation and removal with iron electrocoagulation in groundwater. Environ. Sci. Technol. 2012, 46, 12038–12045. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Clifford, D.A.; Samanta, G. Ferrous and Ferric Ion Generation During Iron Electrocoagulation. Environ. Sci. Technol. 2009, 43, 3853–3859. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Hansen, H.K.; Núñez, P.; Valdés, E. Electrochemical peroxidation using iron nanoparticles to remove arsenic from copper smelter wastewater. Electrochim. Acta 2015, 181, 228–232. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Stefan, M. Arsenic oxidation by UV radiation combined with hydrogen peroxide. Water Sci. Technol. 2010, 61, 339–344. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Stefan, M. UV/H2O2 oxidation of arsenic and terbuthylazine in drinking water. Environ. Monit. Assess. 2014, 186, 1311–1316. [Google Scholar] [CrossRef]
- Yang, H.; Lin, W.-Y.; Rajeshwar, K. Homogeneous and heterogeneous photocatalytic reactions involving As(III) and As(V) species in aqueous media. J. Photochem. Photobiol. A Chem. 1999, 123, 137–143. [Google Scholar] [CrossRef]
- Lescano, M.; Zalazar, C.; Cassano, A.; Brandi, R. Kinetic modeling of arsenic (III) oxidation in water employing the UV/H2O2 process. Chem. Eng. J. 2012, 211–212, 360–368. [Google Scholar] [CrossRef]
- Lescano, M.R.; Zalazar, C.S.; Cassano, A.E.; Brandi, R.J. Arsenic (iii) oxidation of water applying a combination of hydrogen peroxide and UVC radiation. Photochem. Photobiol. Sci. 2011, 10, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M.; Gebologlu, U.; Ulu, F.; Oncel, S.; Demirbas, E. Removal of arsenic from drinking water by the electrocoagulation using Fe and Al electrodes. Electrochim. Acta 2011, 56, 5060–5070. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Ulu, F. Evaluation of operating parameters with respect to charge loading on the removal efficiency of arsenic from potable water by electrocoagulation. J. Environ. Chem. Eng. 2016, 4, 1484–1494. [Google Scholar] [CrossRef]
- Delaire, C.; Amrose, S.; Zhang, M.; Hake, J.; Gadgil, A. How do operating conditions affect As(III) removal by iron electrocoagulation? Water Res. 2017, 112, 185–194. [Google Scholar] [CrossRef]
- Amrose, S.; Gadgil, A.; Srinivasan, V.; Kowolik, K.; Muller, M.; Huang, J.; Kostecki, R. Arsenic removal from groundwater using iron electrocoagulation: Effect of charge dosage rate. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2013, 48, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, N.; Kojima, T.; Basha, C.A.; Srinivasakannan, C. Removal of arsenic from aqueous solution using electrocoagulation. J. Hazard. Mater. 2009, 167, 966–969. [Google Scholar] [CrossRef]
- Arienzo, M.; Chiarenzelli, J.; Scrudato, R. Removal of arsenic in aqueous solution by electrochemical Peroxidation. Fresenius Environ. Bull. 2001, 10, 731–735. [Google Scholar]
- Gutiérrez, C.; Hansen, H.K.; Nuñez, P.; Jensen, P.E.; Ottosen, L.M. Electrochemical peroxidation as a tool to remove arsenic and copper from smelter wastewater. J. Appl. Electrochem. 2010, 40, 1031–1038. [Google Scholar] [CrossRef]
- Aoudj, S.; Khelifa, A.; Zemmouri, H.; Hamadas, I.; Yatoui, S.; Zabchi, N.; Drouiche, N. Degradation of EDTA in H2O2-containing wastewater by photo-electrochemical peroxidation. Chemosphere 2018, 208, 984–990. [Google Scholar] [CrossRef]
- Farhadi, S.; Aminzadeh, B.; Torabian, A.; Khatibikamal, V.; Alizadeh Fard, M. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard. Mater. 2012, 219–220, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Altin, A. An alternative type of photoelectro-Fenton process for the treatment of landfill leachate. Sep. Purif. Technol. 2008, 61, 391–397. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Boye, B.; Dieng, M.M.; Brillas, E. Electrochemical degradation of 2,4,5-trichlorophenoxyacetic acid in aqueous medium by peroxi-coagulation. Effect of pH and UV light. Electrochim. Acta 2003, 48, 781–790. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Nienhauser, A.B.; Fajardo, A.S.; Bansal, R.; Coonrod, C.L.; Fortner, J.D.; Marcos-Hernández, M.; Rogers, T.; Villagran, D.; Wong, M.S.; et al. Disparities between experimental and environmental conditions: Research steps toward making electrochemical water treatment a reality. Curr. Opin. Electrochem. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Stirling, R.; Walker, W.S.; Westerhoff, P.; Garcia-Segura, S. Techno-economic analysis to identify key innovations required for electrochemical oxidation as point-of-use treatment systems. Electrochim. Acta 2020, 338, 135874. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Liebman, J.F. The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2+). J. Phys. Chem. 1984, 88, 99–101. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Klaening, U.K.; Bielski, B.H.J.; Sehested, K. Arsenic(IV). A pulse-radiolysis study. Inorg. Chem. 1989, 28, 2717–2724. [Google Scholar] [CrossRef]
- Bataineh, H.; Pestovsky, O.; Bakac, A. PH-induced mechanistic changeover from hydroxyl radicals to iron(iv) in the Fenton reaction. Chem. Sci. 2012, 3, 1594–1599. [Google Scholar] [CrossRef]
- Hug, S.J.; Leupin, O. Iron-catalyzed oxidation of Arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environ. Sci. Technol. 2003, 37, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Cui, Y.; Lei, L.; Gao, Y.; Zhou, Z.; Li, Y.; Shi, X. An Electrochemical Process Comparison of As(III) in Simulated Groundwater at Low Voltage in Mixed and Divided Electrolytic Cells. Water 2020, 12, 1126. [Google Scholar] [CrossRef] [Green Version]
- Cabrera Reina, A.; Santos-Juanes, L.; García Sánchez, J.L.; Casas López, J.L.; Maldonado Rubio, M.I.; Li Puma, G.; Sánchez Pérez, J.A. Modelling the photo-Fenton oxidation of the pharmaceutical paracetamol in water including the effect of photon absorption (VRPA). Appl. Catal. B Environ. 2015, 166–167, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Grimes, D.R.; Robbins, C.; O’Hare, N.J. Dose modeling in ultraviolet phototherapy. Med. Phys. 2010, 37, 5251–5257. [Google Scholar] [CrossRef]
- Addy, S.A. Electrochemical Arsenic Remediation for Rural Bangladesh. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2009. [Google Scholar]
- Wang, Z.; Bush, R.T.; Liu, J. Arsenic(III) and iron(II) co-oxidation by oxygen and hydrogen peroxide: Divergent reactions in the presence of organic ligands. Chemosphere 2013, 93, 1936–1941. [Google Scholar] [CrossRef]
- Wang, Z.; Bush, R.T.; Sullivan, L.A.; Liu, J. Simultaneous Redox Conversion of Chromium(VI) and Arsenic(III) under Acidic Conditions. Environ. Sci. Technol. 2013, 47, 6486–6492. [Google Scholar] [CrossRef]
- Wang, Z.; Bush, R.T.; Sullivan, L.A.; Chen, C.; Liu, J. Selective oxidation of arsenite by peroxymonosulfate with high utilization efficiency of oxidant. Environ. Sci. Technol. 2014, 48, 3978–3985. [Google Scholar] [CrossRef]






| Treatment | tEC, WHO | As Removal Capacity | Operating Cost | ||||
|---|---|---|---|---|---|---|---|
| min | µg As removed/Coulomb | µg As removed/mg Fe | Energy, $/m3 | Electrode, $/m3 | H2O2, $/m3 | Total, $/m3 | |
| EC | 27.6 | 16.6 | 57.3 | 0.0014 | 0.0048 | - | 0.0062 |
| ECP | 6.7 | 63.0 | 217.8 | 0.0003 | 0.0012 | 0.0045 | 0.0060 |
| PECP | 4.9 | 111.1 | 384.0 | 0.2436 | 0.0009 | 0.0045 | 0.2490 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montefalcon, M.F.V.; Chiong, M.R., III; Resurreccion, A.C.; Garcia-Segura, S.; Ocon, J.D. Arsenic Removal by Advanced Electrocoagulation Processes: The Role of Oxidants Generated and Kinetic Modeling. Catalysts 2020, 10, 928. https://doi.org/10.3390/catal10080928
Montefalcon MFV, Chiong MR III, Resurreccion AC, Garcia-Segura S, Ocon JD. Arsenic Removal by Advanced Electrocoagulation Processes: The Role of Oxidants Generated and Kinetic Modeling. Catalysts. 2020; 10(8):928. https://doi.org/10.3390/catal10080928
Chicago/Turabian StyleMontefalcon, Micah Flor V., Meliton R. Chiong, III, Augustus C. Resurreccion, Sergi Garcia-Segura, and Joey D. Ocon. 2020. "Arsenic Removal by Advanced Electrocoagulation Processes: The Role of Oxidants Generated and Kinetic Modeling" Catalysts 10, no. 8: 928. https://doi.org/10.3390/catal10080928