Enhancement of Photocatalytic Activities with Nanosized Polystyrene Spheres Patterned Titanium Dioxide Films for Water Purification
Abstract
:1. Introduction
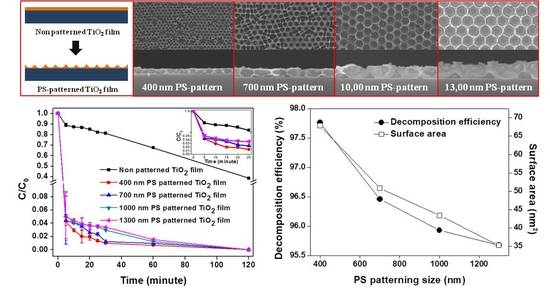
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Polystyrene (PS) Spheres
3.2. Preparation of the TiO2Sol–Gel
3.3. Synthesis of Nonpatterned and PS-Patterned TiO2Thin Films
3.4. The Methods of Photocatalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thakur, M.; Sharma, G.; Ahamad, T.; Ghfar, A.A.; Pathania, D.; Naushad, M. Efficient photocatalytic degradation of toxic dyes from aqueous environment using gelatin-Zr(IV) phosphate nanocomposite and its antimicrobial activity. Colloids Surf. B 2017, 157, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Malathi, A.; Daneshvar, E.; Kollu, P.; Bhatnagar, A. Photocatalytic degradation of toxic aquatic pollutants by novel magnetic 3D-TiO2@HPGA nanocomposite. Sci. Rep. 2018, 8, 15531–15545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanker, U.; Jassal, V.; Rani, M. Green synthesis of iron hexacyanoferrate nanoparticles: Potential candidate for the degradation of toxic PAHs. J. Environ. Chem. Eng. 2017, 5, 4108–4120. [Google Scholar] [CrossRef]
- Sajid, M.M.; Amin, N.; Shad, N.A.; Khan, S.B.; Javed, Y.; Zhang, Z. Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater. Sci. Eng. B 2019, 242, 83–89. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Y.; Yang, C.-L. Selective catalytic reduction of NO by NH3 in flue gases over a Cu-V/Al2O3 catalyst at low temperature. Environ. Eng. Sci. 2009, 26, 1429–1434. [Google Scholar] [CrossRef]
- Wu, Y.; Jing, X.; Gao, C.; Huang, Q.; Cai, P. Recent advances in microbial electrochemical system for soil bioremediation. Chemosphere 2018, 211, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Role of titanium dioxide (TiO2) structural design/morphology in photocatalytic air purification. Appl. Catal. B Environ. 2020, 269, 118735–118752. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic investigation of the anatase-to-rutile transformation of sol-gel-synthesized TiO2 photocatalyst. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, K.; Zhou, S. Determination of estrogenic steroids and microbial and photochemical degradation of 17α-ethinylestradiol(EE2) in lake surface water, a case study. Environ. Sci. Process. Impacts 2013, 15, 1529–1535. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Chen, P.; Lv, W.; Liu, G. In-situ stabilizing surface oxygen vacancies of TiO2 nanowire array photoelectrode by N-doped carbon dots for enhanced photoelectrocatalytic activities under visible light. J. Catal. 2020, 382, 212–227. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Q.; Deng, X.; Wang, P.; Liu, H. A facile and novel strategy to synthesize reduced TiO2 nanotubes photoelectrode for photoelectrocatalytic degradation of diclofenac. Chemosphere 2016, 144, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Sopha, H.; Tesar, K.; Knotek, P.; Jager, A.; Hromadko, L.; Macak, J.M. TiO2 nanotubes grown on Ti substrates with different microstructure. J. Mater. Res. Bull. 2018, 103, 197–204. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.S.; Smith, Y.R.; Misra, M.; Subramanian, V.R. Electrochemically assisted photocatalytic degradation of methyl orange using anodized titanium dioxide nanotubes. Appl. Catal. B Environ. 2008, 84, 372–378. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, K.; Weng, W. SiO2/TiO2 nanocomposite films on polystyrene for light-induced cell detachment application. ACS Appl. Mater. Interfaces 2017, 9, 2130–2137. [Google Scholar] [CrossRef]
- Riaz, S.; Ashraf, M.; Hussain, T.; Hussain, M.T.; Younus, A. Fabrication of robust multifaceted textiles by application of functionalized TiO2 nanoparticles. Colloids Surf. A 2019, 581, 123799–123811. [Google Scholar] [CrossRef]
- Ivanova, I.; Schneider, J.; Gutzmann, H.; Kliemann, J.-O.; Gartner, F.; Klassen, T.; Bahnemann, D.; Mendive, C.B. Photocatalytic degradation of oxalic and dichloroacetic acid on TiO2 coated metal substrates. Catal. Today 2013, 209, 84–90. [Google Scholar] [CrossRef]
- Nam, S.H.; Kim, T.K.; Boo, J.-H. Physical property and photo-catalytic activity of sulfur doped TiO2 catalysts responding to visible light. Catal. Today 2012, 185, 259–262. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.T.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.C.; Hou, Y.; Fu, X. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 2005, 21, 2552–2559. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Fabrication of porous 3D flower-like Ag/ZnO heterostructure composite with enhanced photocatalytic performance. Appl. Surf. Sci. 2015, 332, 32–39. [Google Scholar] [CrossRef]
- Roy, P.; Dey, T.; Lee, K.; Kim, D.; Fabry, B.; Schmuki, P. Size-selective separation of macromolecules by nanochannel titania membrane with self-cleaning (declogging) ability. J. Am. Chem. Soc. 2010, 132, 7893–7895. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Nishimoto, S.; Murakami, T.; Fujishima, A. Efficient photocatalytic degradation of gaseous acetaldehyde by highly ordered TiO2 nanotube arrays. Environ. Sci. Technol. 2008, 42, 8547–8551. [Google Scholar] [CrossRef]
- Liang, H.-C.; Li, X.-Z. Effects of structure of anodic TiO2 nanotube arrays on photocatalytic activity for the degradation of 2,3-dichlorophenol in aqueous solution. J. Hazard. Mater. 2009, 162, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef]
- Zhuang, H.-F.; Lin, C.-J.; Lai, Y.-K.; Sun, L.; Li, J. Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Kar, A.; Subramanian, V.R. Investigation of physicochemical parameters that influence photocatalytic degradation of methyl orange over TiO2 nanotubes. Ind. Eng. Chem. Res. 2009, 48, 10268–10276. [Google Scholar] [CrossRef]
- Ku, Y.; Fan, Z.-R.; Chou, Y.-C.; Wang, W.-Y. Effects of TiO2 nanotube array dimension and annealing temperature on the acid red 4 degradation in aqueous solution by photocatalytic process. Water Sci. Technol. 2010, 61, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.; Roy, P.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Formation of a non-thickness-limited titanium dioxide mesosponge and its use in dye-sensitized solar cells. Angew. Chem. 2009, 121, 9490–9493. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Roy, P.; Paramasivam, I.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Anodic formation of thick anatase TiO2 mesosponge layers for high-efficiency photocatalysis. J. Am. Chem. Soc. 2010, 132, 1478–1479. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals. Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Ling, H.; Kim, K.D.; Liu, Z.; Shi, J.; Zhu, X.; Huang, J. Photocatalytic degradation of phenol in water on as-prepared and surface modified TiO2 nanoparticles. Catal. Today 2015, 258, 96–102. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Q.; Yuan, S.; Ohno, T. Synthesis high specific surface area nanotube g-C3N4 with two-step condensation treatment of melamine to enhance photocatalysis properties. RSC Adv. 2015, 5, 4026–4029. [Google Scholar] [CrossRef] [Green Version]
- Doong, R.-A.; Chang, S.-M.; Hung, Y.-C.; Kao, I.-L. Preparation of highly ordered titanium dioxide porous films: Characterization and photocatalytic activity. Sep. Purif. Technol. 2007, 58, 192–199. [Google Scholar] [CrossRef]
- Motola, M.; Dworniczek, E.; Satrapinskyy, L.; Chodaczek, G.; Grzesiak, J.; Gregor, M.; Plecenik, T.; Nowicka, J.; Plesch, G. UV light-induced photocatalytic, antimicrobial, and antibiofilm performance of anodic TiO2 nanotube layers prepared on titanium mesh and Ti sputtered on silicon. Chem. Pap. 2019, 73, 1163–1172. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.J.; Lee, J.W.; Na, Y.H.; Boo, J.-H. Enhancement of Photocatalytic Activities with Nanosized Polystyrene Spheres Patterned Titanium Dioxide Films for Water Purification. Catalysts 2020, 10, 886. https://doi.org/10.3390/catal10080886
Seo HJ, Lee JW, Na YH, Boo J-H. Enhancement of Photocatalytic Activities with Nanosized Polystyrene Spheres Patterned Titanium Dioxide Films for Water Purification. Catalysts. 2020; 10(8):886. https://doi.org/10.3390/catal10080886
Chicago/Turabian StyleSeo, Hyeon Jin, Ji Won Lee, Young Hoon Na, and Jin-Hyo Boo. 2020. "Enhancement of Photocatalytic Activities with Nanosized Polystyrene Spheres Patterned Titanium Dioxide Films for Water Purification" Catalysts 10, no. 8: 886. https://doi.org/10.3390/catal10080886