Synthesis of HZSM-5 Rich in Paired Al and Its Catalytic Performance for Propane Aromatization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Catalysts
2.2. Al distribution in the ZSM-5 Framework
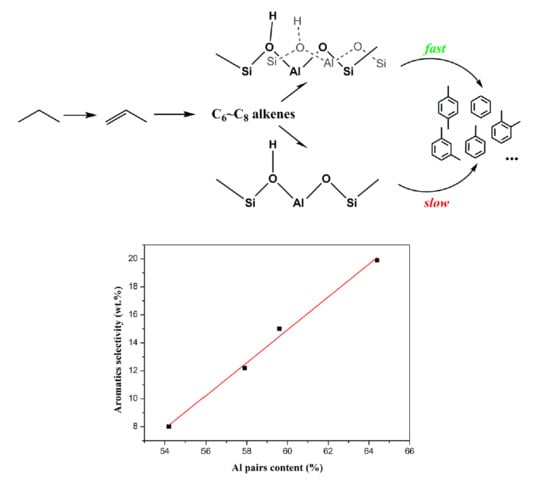
2.3. Catalytic Performance
2.3.1. Catalytic Performance of HZSM-5 Zeolites in the Fixed-Bed Reactor
2.3.2. Micro Reactor Activity Estimation
2.3.3. Pulse Experiment of Propane Aromatization
2.3.4. Operando Diffuse Reflectance Ultraviolet-Visible (DR UV-vis) Spectra Experiment
2.4. Coke Analysis
2.5. DFT Calculations
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalysis tests
3.3.1. Propane Conversion in a Fixed-Bed Reactor
3.3.2. Propane Conversion in a Micro Reactor
3.3.3. Pulse Reaction Test of Propane Conversion
3.4. DFT Calculation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhan, A.; Delgass, W.N. Propane Aromatization over HZSM-5 and Ga/HZSM-5 Catalysts. Catal. Rev. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, L.; Yang, G.; Tsubaki, N. One-pass selective conversion of syngas to paraxylene. Chem. Sci. 2017, 8, 7941–7946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Al-Khattaf, S. Physicochemical properties and catalytic performance of galloaluminosilicate in aromatization of lower alkanes: A comparative study with Ga/HZSM-5. J. Porous Mater. 2012, 19, 943–960. [Google Scholar] [CrossRef]
- Su, X.; Wang, G.; Bai, X.; Wu, W.; Xiao, L.; Fang, Y.; Zhang, J. synthesis of nanosized HZSM-5 zeolites isomorphously substituted by gallium and their catalytic performance in the aromatization. Chem. Eng. J. 2016, 293, 365–375. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Sivadinarayana, C.; Kinage, A.K.; Devadas, P.; Guisnet, M. H-Gallosilicate (MFI) propane aromatization catalyst Influence of calcination temperature on acidity, activity and deactivation due to coking. Appl. Catal. A Gen. 1996, 136, 125–142. [Google Scholar] [CrossRef]
- Montes, A.; Giannetto, G. A new way to obtain acid or bifunctional catalysts: V. Considerations on bifunctionality of the propane aromatization reaction over [Ga,Al]-ZSM-5 catalysts. Appl. Catal. A Gen. 2000, 197, 31–39. [Google Scholar] [CrossRef]
- Rodrigues, V.O.; Faro Júnior, A.C. On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts. Appl. Catal. A Gen. 2012, 435, 68–77. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Kinage, A.K.; Sivadinarayana, C.; Devadas, P.; Sansare, S.D.; Guisnet, M. H-Gallosilicate (MFI) Propane Aromatization Catalyst: Influence of Si/Ga Ratio on Acidity, Activity and Deactivation Due to Coking. J. Catal. 1996, 158, 34–50. [Google Scholar] [CrossRef]
- Han, J.; Jiang, G.; Han, S.; Liu, J.; Zhang, Y.; Liu, Y.; Wang, R.; Zhao, Z.; Xu, C.; Wang, Y.; et al. The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics. Catalysts 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Al-Yassir, N.; Akhtar, M.N.; Ogunronbi, K.; Al-Khattaf, S. Synthesis of stable H-galloaluminosilicate MFI with hierarchical pore architecture by surfactant-mediated base hydrolysis, and their application in propane aromatization. J. Mol. Catal. A Chem. 2012, 360, 1–15. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Aittaleb, D.; Doyemet, Y.J. Conversion of light alkanes into aromatic hydrocarbons: VI. Aromatization of C2-C4 alkanes on H-ZSM-5—Reaction mechanisms. Appl. Catal. A Gen. 1992, 87, 255–270. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Iglesia, E. Isotopic Tracer Studies of Propane Reactions on H− ZSM5 Zeolite. J. Phys. Chem. B 1998, 102, 9284–9289. [Google Scholar] [CrossRef] [Green Version]
- Meriaudeau, P.; Naccache, C. Further Evidence on the Change of Acid Properties of H-ZSM-5 by Ga and Pt. J. Catal. 1995, 157, 283–288. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Meitzner, G.D.; Iglesia, E. Structure and Density of Active Zn Species in Zn/H-ZSM5 Propane Aromatization Catalysts. J. Catal. 1998, 179, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Dedecek, J.; Kaucky, D.; Wichterlova, B.; Gonsiorova, O. Co2+ ions as probes of Al distribution in the framework of zeolites. ZSM-5 study. Phys. Chem. Chem. Phys. 2002, 4, 5406–5413. [Google Scholar] [CrossRef]
- Liang, T.; Chen, J.; Qin, Z.; Li, J.; Wang, P.; Wang, S.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Conversion of Methanol to Olefins over H-ZSM-5 Zeolite: Reaction Pathway Is Related to the Framework Aluminum Siting. ACS Catal. 2016, 6, 7311–7325. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Chen, Y.; Dong, M.; Li, J.; Zhang, K.; Liu, P.; Wang, J.; Fan, W. Relation of Catalytic Performance to the Aluminum Siting of Acidic Zeolites in the Conversion of Methanol to Olefins, Viewed via a Comparison between ZSM-5 and ZSM-11. ACS Catal. 2018, 8, 5485–5505. [Google Scholar] [CrossRef]
- Biligetu, T.; Wang, Y.; Nishitob, T.; Otomo, R.; Park, S.; Mochizuki, H.; Kondo, J.N.; Tatsumi, T.; Yokoi, T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols. J. Catal. 2017, 353, 1–10. [Google Scholar] [CrossRef]
- Sazama, P.; Dedecek, J.; Gábová, V.; Wichterlová, B.; Spoto, G.; Bordiga, S. Effect of aluminium distribution in the framework of ZSM-5 on hydrocarbon transformation. Cracking of 1-butene. J. Catal. 2008, 254, 180–189. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Bjorgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefinic species. J. Catal. 2007, 249, 195–207. [Google Scholar] [CrossRef]
- Song, C.; Chu, Y.; Wang, M.; Shi, H.; Zhao, L.; Guo, X.; Yang, W.; Shen, J.; Xue, N.; Peng, L.; et al. Cooperativity of adjacent Brønsted acid sites in MFI zeolite channel leads to enhanced polarization and cracking of alkanes. J. Catal. 2017, 349, 163–174. [Google Scholar] [CrossRef]
- Tabor, E.; Bernauer, M.; Wichterlová, B.; Dedecek, J. Enhancement of propene oligomerization and aromatization by proximate protons in zeolites; FTIR study of the reaction pathway in ZSM-5 . Catal. Sci. Technol. 2019, 9, 4262–4275. [Google Scholar] [CrossRef]
- Li, J.; Ma, H.; Chen, Y.; Xu, Z.; Li, C.; Ying, W. Conversion of methanol to propylene over hierarchical HZSM-5: The effect of Al spatial distribution. Chem. Commun. 2018, 54, 6032–6035. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Zhang, L.; Qin, Z.; Dong, M.; Li, J.; Wang, J.; Fan, W. Insight into the effect of incorporation of boron into ZSM-11 on its catalytic performance for conversion of methanol to olefins. Catal. Sci. Technol. 2017, 7, 4766–4779. [Google Scholar] [CrossRef]
- Dai, W.; Wu, G.; Li, L.; Guan, N.; Hunger, M. Mechanisms of the Deactivation of SAPO-34 Materials with Different Crystal Sizes Applied as MTO Catalysts. ACS Catal. 2013, 3, 588–596. [Google Scholar] [CrossRef]
- Bjørgen, M.; Bonino, F.; Kolboe, S.; Lillerud, K.-P.; Zecchina, A.; Bordiga, S. Spectroscopic Evidence for a Persistent Benzenium Cation in Zeolite H-Beta. J. Am. Chem. Soc. 2003, 125, 15863–15868. [Google Scholar] [CrossRef]
- Wulfers, M.J.; Jentoft, F.C. Identification of carbonaceous deposits formed on H-mordenite during alkane isomerization. J. Catal. 2013, 307, 204–213. [Google Scholar] [CrossRef]
- Borodina, E.; Sharbini Harun Kamaluddin, H.; Meirer, F.; Mokhtar, M.; Asiri, A.M.; Al Thabaiti, S.A.; Basahel, S.N.; Ruiz-Martinez, J.; Weckhuysen, B.M. Influence of the Reaction Temperature on the Nature of the Active and Deactivating Species during Methanol-to-Olefins Conversion over H-SAPO-34. ACS Catal. 2017, 7, 5268–5281. [Google Scholar] [CrossRef] [Green Version]
- Borodina, E.; Meirer, F.; Lezcano-Gonzalez, I.; Mokhtar, M.; Asiri, A.M.; Al-Thabaiti, S.A.; Basahel, S.N.; Ruiz-Martinez, J.; Weckhuysen, B.M. Influence of the Reaction Temperature on the Nature of the Active and Deactivating Species during Methanol to Olefins Conversion over H-SSZ-13. ACS Catal. 2015, 5, 992–1003. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Wang, S.; Cui, X.; Dong, M.; Wang, G.; Qin, Z.; Wang, J.; Fan, W. Co-reaction of methanol with butene over a high-silica H-ZSM-5 catalyst. J. Catal. 2018, 367, 315–325. [Google Scholar] [CrossRef]
- Song, C.; Liu, K.; Zhang, D.; Liu, S.; Li, X.; Xie, S.; Xu, L. Effect of cofeeding n-butane with methanol on aromatization performance and coke formation over a Zn loaded ZSM-5/ZSM-11 zeolite. Appl. Catal. A Gen. 2014, 470, 15–23. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Jentoft, F.C. Spectroscopic Signatures Reveal Cyclopentenyl Cation Contributions in Methanol-to-Olefins Catalysis. ACS Catal. 2020, 10, 5764–5782. [Google Scholar] [CrossRef]
- Emdadi, L.; Mahoney, L.; Lee, I.C.; Leff, A.C.; Wu, W.; Liu, D.; Nguyen, C.K.; Tran, D.T. Assessment of coke deposits on lamellar metal-modifed MFI zeolites in ethylene transformation to aromatic liquids. Appl. Catal. A Gen. 2020, 595, 117510–117520. [Google Scholar] [CrossRef]
- Maksoud, W.A.; Gevers, L.E.; Vittenet, J.; Ould-Chikh, S.; Telalovic, S.; Bhatte, K.; Abou-Hamad, E.; Anjum, D.H.; Hedhili, M.N.; Vishwanath, V.; et al. A strategy to convert propane to aromatics (BTX) using TiNp 4 grafted at the periphery of ZSM-5 by surface organometallic chemistry. Dalton Trans. 2019, 48, 6611–6620. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, Y.; Du, Y.; Yang, G.; Xie, Z. Similarities and differences between aromatic-based and olefin-based cycles in H-SAPO-34 and H-SSZ-13 for methanol-to-olefins conversion: Insights from energetic span model. Catal. Sci. Technol. 2015, 5, 4354–4364. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Wei, Z.; Qin, Z.; Ma, H.; Dong, M.; Li, J.; Fan, W.; Wang, J. Polymethylbenzene or alkene cycle? theoretical study on their contribution to the process of methanol to olefins over H-ZSM-5 zeolite. J. Phys. Chem. C 2015, 119, 28482–28498. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Qin, Z.; Zhao, T.; Fan, S.; Dong, M.; Li, J.; Fan, W.; Wang, J. Origin and evolution of the initial hydrocarbon pool intermediates in the transition period for the conversion of methanol to olefins over H-ZSM-5 zeolite. J. Catal. 2019, 369, 382–395. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S. Aromatization of propane over GaHMFI catalysts. Reaction scheme, nature of the dehydrogenating species and mode of coke formation. Catal. Today 1996, 31, 275–292. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]














| Catalyst | Crystal System | Lattice Parameters | |||||
|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α | β | γ | ||
| HZ(0) | Orthorhombic | 20.091 | 19.899 | 13.384 | 90° | 90° | 90° |
| HZ(0.4) | Orthorhombic | 20.102 | 19.902 | 13.389 | 90° | 90° | 90° |
| HZ(0.8) | Orthorhombic | 20.090 | 19.905 | 13.389 | 90° | 90° | 90° |
| HZ(1.1) | Orthorhombic | 20.098 | 19.907 | 13.390 | 90° | 90° | 90° |
| Catalysts | Si/Al a | Si/AlF b | AlF/Altotal b (%) | Stotal c (m2 g−1) | Smicro d (m2 g−1) | Vtotal e (m3 g−1) | Vmicro d (m3 g−1) | PSD f (nm) |
|---|---|---|---|---|---|---|---|---|
| HZ(0) | 42 | 43 | 97.2 | 420 | 256 | 0.45 | 0.11 | 0.772~0.879 |
| HZ(0.4) | 37 | 38 | 97.9 | 380 | 257 | 0.34 | 0.11 | 0.772~0.879 |
| HZ(0.8) | 38 | 40 | 95.7 | 363 | 267 | 0.28 | 0.12 | 0.772~0.879 |
| HZ(1.1) | 38 | 39 | 97.6 | 383 | 260 | 0.33 | 0.11 | 0.772~0.879 |
| Catalysts | Acidity by Py-IR (mmol g−1) a | Acidity by NH3-TPD (mmol g−1) b | ||||
|---|---|---|---|---|---|---|
| Brønsted | Lewis | Total | Strong | Weak | Total | |
| HZ(0) | 0.20 | 0.04 | 0.24 | 0.32 | 0.28 | 0.62 |
| HZ(0.4) | 0.22 | 0.04 | 0.26 | 0.37 | 0.27 | 0.64 |
| HZ(0.8) | 0.22 | 0.05 | 0.27 | 0.36 | 0.27 | 0.63 |
| HZ(1.1) | 0.21 | 0.03 | 0.24 | 0.36 | 0.28 | 0.64 |
| Samples | AlF Content (%) | Al Pair Distribution (%) c | Al Content by NMR (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Alsingle a | Alpairs a | (2Co + Na)/Al b | α | β | γ | Al(54) d | Al(56) d | |
| Co/ZSM-5(0) | 45.8 | 54.2 | 0.99 | 14 | 64 | 22 | 31 | 29 |
| Co/ZSM-5(0.4) | 42.1 | 57.9 | 0.99 | 10 | 66 | 24 | 33 | 27 |
| Co/ZSM-5(0.8) | 35.6 | 64.4 | 0.96 | 10 | 65 | 25 | 33 | 27 |
| Co/ZSM-5(1.1) | 40.4 | 59.6 | 0.97 | 7 | 68 | 25 | 31 | 27 |
| Catalysts | Aromatics Concentration (×104) a | Olefin Concentrations a | ||
|---|---|---|---|---|
| Ethene | Propene | Butene | ||
| HZ(0) | 7.71 | 0.924 | 0.159 | 0.015 |
| HZ(0.8) | 14.1 | 0.918 | 0.089 | 0.014 |
| Step | ΔGint≠ (kJ mol−1) | k (s−1) | ΔHint≠ (kJ mol−1) | −TΔSint≠ (kJ mol−1) | ΔGR (kJ mol−1) |
|---|---|---|---|---|---|
| Single AlF | |||||
| TS1 | 176 | 1.12 × 102 | 157 | 19 | −19 |
| TS2 | 158 | 1.75 × 103 | 160 | −2 | 105 |
| TS3 | 156 | 2.06 × 103 | 151 | 5 | 9 |
| TS4 | 166 | 5.11 × 102 | 158 | 8 | −1 |
| AlF pairs | |||||
| TS1 | 171 | 2.50 × 102 | 151 | 20 | −28 |
| TS2 | 145 | 1.03 × 104 | 154 | −9 | 108 |
| TS3 | 149 | 5.34 × 103 | 143 | 6 | 27 |
| TS4 | 159 | 1.38 × 103 | 149 | 10 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Wang, S.; Wang, H.; Wang, P.; Zhang, L.; Qin, Z.; Wang, J.; Zhu, H.; Fan, W. Synthesis of HZSM-5 Rich in Paired Al and Its Catalytic Performance for Propane Aromatization. Catalysts 2020, 10, 622. https://doi.org/10.3390/catal10060622
Shi D, Wang S, Wang H, Wang P, Zhang L, Qin Z, Wang J, Zhu H, Fan W. Synthesis of HZSM-5 Rich in Paired Al and Its Catalytic Performance for Propane Aromatization. Catalysts. 2020; 10(6):622. https://doi.org/10.3390/catal10060622
Chicago/Turabian StyleShi, Dezhi, Sen Wang, Hao Wang, Pengfei Wang, Li Zhang, Zhangfeng Qin, Jianguo Wang, Huaqing Zhu, and Weibin Fan. 2020. "Synthesis of HZSM-5 Rich in Paired Al and Its Catalytic Performance for Propane Aromatization" Catalysts 10, no. 6: 622. https://doi.org/10.3390/catal10060622