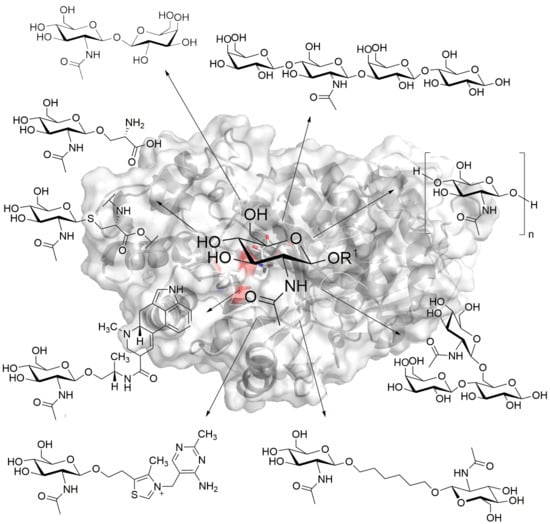
β-N-Acetylhexosaminidases for Carbohydrate Synthesis via Trans-Glycosylation
Abstract
:1. Introduction
2. GH20 β-N-Acetylhexosaminidases
3. Increased Trans-Glycosylation Activity by Reaction Engineering
3.1. Reverse Hydrolysis VS. Trans-Glycosylation
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AnHex (Aspergillus niger CCIM K2) | GalNAc (1500) 2 | 1:1 | GalNAc-α-1,3-GalNAc (I), GalNAc-α-1,6-GalNAc (II) | 3.6 | 35 | 120 | 15 (I + II) 3 | [23] | |
| t-Boc-Ser, t-Boc-Thr (1130) | α-GalNAc (1130) | 1:1 | GalNAc-α-O-Ser (I), GalNAc-α-O-Thr (II) | 4.8 | 35 | 168 | 7.4 (I), 3.6 (II) 3,4 | [23] | |
| AoHex (Aspergillus oryzae) | GlcNAc-β-N-Ac (1115), GlcNAc-β-N-Pr (754) | GlcNAc (113/75) | 10:1 | GlcNAc-β-1,6-GlcNAc-β-N-Ac (I), GlcNAc-β-1,6-GlcNAc-β-N-Pr (II) | 5.0 | 37 | 120, 168 | 13 (I), 8 (II) 3,5 | [49] |
| AoHex (Aspergillus oryzae CCF 1066) | GlcNAc (1500) 2 | 1:1 | GlcNAc-β-1,6-GlcNAc | 4.9 | 39 | 144 | 1.9–14.5 3,6 | [46] | |
| Alcohols and diols | GlcNAc | n.a. | Alkyl glycosides 7 (β-O) | 4.2 | 37 | 72 | n.a. 6 | [46] | |
| AoHex (Aspergillus oryzae RIB40) | Lac (1218) | GlcNAc (565) | 2:1 | LNT II (β-1,3) (I), GlcNAc-β-1,6-Lac (II) | 6.0 | 45 | 96 | 0.36 (I), 0.72 (II) 3 | [50] |
| LnbB (Bifidobacterium bifidum JCM1254) | Lac (1000) | LNB (100) | 10:1 | LNT (β-1,3), one unidentified regioisomer 8 | 4.5 | 40 | n.a. | n.d. 9 | [51] |
| BcHex (Bacillus circulans) | Man (9436) | GlcNAc (2260) | 4:1 | GlcNAc-β-1,2-Man (I), GlcNAc-β-1,6-Man (II) | 5.0 | 37 | 360 | 0.3 (I), 2 (II) 3 | [45] |
| PfHex (Penicillium funiculosus CCF 1994) | GlcNAc (1031) 2 | 1:1 | GlcNAc-β-1,3-GlcNAc (I), GlcNAc-β-1,4-GlcNAc (II), GlcNAc-β-1,6-GlcNAc (III) | 5.0 | 37 | 192 | 3.8 (I), 1.7 (II), 10 (III) 3,5 | [52] | |
| PgHex (Phoma glomerata) | MeOH (4944) | GlcNAc, GalNAc (90 each) | 55:1 | GlcNAc-β-O-Me (I), GalNAc-β-O-Me (II) | 7.4 | 37 | 168 | 37.8 (I), 46 (II) 9 | [47] |
3.1.1. p-Nitrophenyl Activated Donors
3.1.2. Other Activated Donors
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AoHex (A. oryzae) | 6-benzyl-Gal-β-S-Et (118, 73) | pNP-GlcNAc (96), pNP-GalNAc (67) | 1:1 | GlcNAc-β-1,3-6-benzyl-Gal-β-S-Et (I), GalNAc-β-1,3-6-benzyl–Gal-β-S-Et (II) | 7.0 | 25 | 2.5, 30 | 2.8 (I), 5.7 (II) 2 | [53] |
| 3 | Glc-α-O-pNP (41), GlcNAc-α-O-pNP (124) | 6-SO3-GlcNAc-β-O-pNP (10/25) | 4:1, 5:1 | 6-SO3-GlcNAc-β-1,4-Glc-α-O-pNP (I), 6-SO3-GlcNAc-β-1,4-GlcNAc-α-O-pNP (II) | 6.0 | 35 | 148, 168 | 35 (I), 94 (II) 2,4 | [54] |
| 3 | 6-SO3-GlcNAc-β-O-pNP (190) | pNP-GlcNAc (30) | 6:1 | GlcNAc-β-1,4-6-SO3-GlcNAc-β-O-pNP | 6.0 | 35 | 7 | 13.4 2 | [54] |
| 3 | GlcNAc (1157/1112) | pNP-GlcNAc (117/111) | 10:1 | GlcNAc-β-1,4-GlcNAc (I), GlcNAc-β-1,6-GlcNAc (II) | 6.5 | 30 | 40, 215 | 55 (I) 5, 22 (II) 2 | [55] |
| 3 | GlcNAc-α-O-Me (531), GlcNAc-β-O-Me (567) | pNP-GlcNAc (104/97) | 5:1, 6:1 | GlcNAc-β-1,4-GlcNAc-α-O-Me (I), GlcNAc-β-1,4-GlcNAc-β-O-Me (II) | 6.5 | 30 | 120, 48 | 51 (I), 24 (II) 2 | [55] |
| 3 | α-Glc 6, Glc-α-O-R; α-GlcNAc, GlcNAc-α-O-R (each ca. 1300) 7 | 6-SO3-GlcNAc-β-O-pNP (250) | 5:1 | 6-SO3-GlcNAc-β-1,4-Glc-α-O-R (I = R1, II = R3, III = R6), 6-SO3-GlcNAc-β-1,4-GlcNAc-α-R (IV = R5, V = R1, VI = R2, VII = R3, VIII = R4) 7 | 6.0 | 35 | 120–144 | 17 (I), 34 (II), 36 (III), 38 (IV), 51 (V), 87 (VI), 92 (VII), 93 (VIII) 2 | [78] |
| 3 | Glc-α-O-R3, GlcNAc-α- O-R3, GlcNAc, Glc-α-O-R1, Gal-α-O-R3 (each ca. 1700) 7 | 6-SO3-GlcNAc-β-O-pNP (178) | 10:1 | 6-SO3-GlcNAc-β-1,4-Glc-α-O-R3 (I), 6-SO3-GlcNAc-β-1,4-GlcNAc-α-O-R (II = R3, III = R5, IV = R1), 6-SO3-GlcNAc-β-1,3/6-Gal-α-O-R3 (V) 7 | 6.0 | 35 | 74, 66, 370, 71, 48 | 38 (I), 92 (II), 38 (III), 17 (IV), 25 (V) 2 | [79] |
| GlcNAc-β-N-Ac (206) | pNP-GlcNAc (21) | 10:1 | GlcNAc-β-1,4-GlcNAc-β-N-Ac | 5.0 | 37 | 4 | 17 2,12 | [49] | |
| 3 | Glc-β-O-Me, Glc-α-O-Me (603 each) | pNP-GalNAc, pNP-GlcNAc (60) | 10:1 | GalNAc-β-1,3/4-Glc-β-O-Me (I = β-1,3, II = β-1,4), GalNAc-β-1,4/6-Glc-α-O-Me (III = β-1,4, IV = β-1,6), GlcNAc-β-1,3/4-Glc-β-O-Me (V = β-1,3, VI = β-1,4), GlcNAc-β-1,4/6-Glc-α-O-Me (VII = β-1,4, VII = β-1,6) | 6.5 | 28 | 24 | 49 (I + II), 36 (II I+ IV), 23 (V + VI), 17 (VII + VIII) 2 | [80] |
| 3 | GlcNAc-β-O-Me, GlcNAc-α-O-Me (568 each) | pNP-GalNAc (104) | 6:1 | GalNAc-β-1,4/6-GlcNAc-α-O-Me (I = β-1,4, II = β-1,6), GalNAc-β-1,4-GlcNAc-β-O-Me (III), one unidentified isomer | 4.5 | 30 | 96 | 89 (I + II) 2 | [81] |
| 3 | GlcNAc, GlcNAc-β-O-Me, GlcNAc-α-O-Me (772 each) | pNP-GlcNAc (76) | 10:1 | GlcNAc-β-1,4/6-GlcNAc (I = β-1,4, II = β-1,6), GlcNAc-β-1,4-GlcNAc-α-O-Me (III), GlcNAc-β-1,4-GlcNAc-β-O-Me (IV), one unidentified isomer | 6.5 | 30 | n.a. | 55 (I) 8, 22 (II) 8, 55 (III), 24 (IV) 2 | [82] |
| 3 | Man (3996) | pNP-GlcNAc (38 + 29) 9 | 105:1 | GlcNAc-β-1,1/3/4/6-Man (I = β-1,1, II = β-1,3, III= β-1,4, IV = β-1,6) | 5.0 | 37 | 4 | 24.6 (I + II + III + IV) 2 | [83] |
| 3 | GalNAc (988/983) | pNP-GlcNAc (140), pNP-GalNAc (141) | 7:1 | GlcNAc-β-1,6-GalNAc (I), GlcNAc-β-1,4-GlcNAc (II), GalNAc-β-1,6-GalNAc (III) | 6.5, 4.5 | 30 | 30, 47 | 26 (I) 5, 38 (III) 2 | [84] |
| AoHex (A. oryzae CCF 1066) | GlcNAc-β-linker-β-GlcNAc (315, 118) | pNP-GlcNAc (146), pNP-GalNAc (292) | 2:1, 1:2 | GlcNAc-β-1,4-GlcNAc-β-linker-β-GlcNAc (I), GalNAc-β-1,4-GlcNAc-β-linker-β-GlcNAc (II) | 5.0 | 35 | 4.5 | 7 (I), 6 (II) 2 | [85] |
| GlcNAc (230) | pNP-GalNAc (60) | 4:1 | GalNAc-β-1,4-GlcNAc | 5.0 | 37 | 1 | 84.5 2 | [86] | |
| GlcNAc-α-O-R3 (115), Gal-β-1,4-GlcNAc-α- O-R3 (83) 7 | pNP-GalNAc (29) | 4:1, 3:1 | GalNAc-β-1,4-GlcNAc-α-O-R3 (I), GalNAc-β-1,6-GalNAc-β-1,4-GlcNAc-α-O-R3 (II) 7 | 5.0 | 37 | 4, 1.5 | 78 (I), 17 (II) 2 | [87] | |
| GlcNAc-β-1,4-ManNAc (96) | pNP-GalNAc (67) | 1:1 | GalNAc-β-1,4-GlcNAc-β-1,4-ManNAc | 5.0 | 37 | 2 | 41 2,10 | [88] | |
| Gal (223), GalNAc (197), Lac (189) | pNP-GlcNAc (28/38/24) | 8:1, 5:1 | GlcNAc-β-1,1-Gal (I), GlcNAc-β-1,4-GlcNAc-β-1,1-Gal (II), GlcNAc-β-1,6-GalNAc (III), Lac-α/β-1,1-GlcNAc | 5.0 | 37 | 4.5, 5 | 17 (I), 7 (II), 14 (III) 2 | [89] | |
| GlcNAc-β-1,4-ManNAc (104/96) | pNP-GlcNAc (65), pNP-GalNAc (67) | 2:1, 1:1 | GlcNAc-β-1,4-GlcNAc-β-1,4-ManNAc (I), GalNAc-β-1,4-GlcNAc-β-1,4-ManNAc (II) | 5.0 | 37 | 0.8, 2 | 36 (I), 41 (II) 2,10 | [90] | |
| pNP-GlcNAc (24) | pNP-GlcNPr (46) | 1:2 | GlcNPr-β-1,4-GlcNPr-β-O-pNP (I), GlcNPr-β-1,4-GlcNAc-β-O-pNP (II) | 5.0 | 37 | 15.5 | 4.3 (I), 1.8 (II) 2,11 | [61] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AfHex (Aspergillus flavofurcatis CCF 3061) | Gal (223), GalNAc (197), Lac (189) | pNP-GlcNAc (28/38/24) | 8:1, 5:1 | GlcNAc-β-1,1-Gal (I), GlcNAc-β-1,4-GlcNAc-β-1,1-Gal (II), GlcNAc-β-1,6-GalNAc (III), Lac-β-1,1-GlcNAc (IV), Lac-α-1,1-GlcNAc (V) | 5.0 | 37 | 4.5, 5, 7 | 22 (I) 2, 23.5 (II) 2, 20 (III) 2, 10 (IV) 2,3, 9 (V) 2,3 | [89] |
| AtHex (Aspergillus tamarii CCF 1665) | Gal (223), GalNAc (197), Lac (189) | pNP-GlcNAc (28/38/24) | 8:1, 5:1 | GlcNAc-β-1,1-Gal (I), GlcNAc-β-1,4-GlcNAc-β-1,1-Gal (II), GlcNAc-β-1,6-GalNAc (III), Lac-α/β-1,1-GlcNAc 4 | 5.0 | 37 | 4.5, 5 | 12 (I), 6 (II), 17.5 (III) 2 | [89] |
| PbHex (Penicillium brasilianum CCF 2155) | 6-Ac-GlcNAc (380), GlcNAc (452) | pNP-GlcNAc (42), 6-Ac-GlcNAc-β-O-pNP (40) | 9:1, 11:1 | GlcNAc-β-1,4-6-O-Ac-GlcNAc (I), 6-O-Ac-GlcNAc-β-1,4-GlcNAc (II) | 5.5 | 37 | 2, 4.5 | 16.5 (I), 21 (II) 2,5 | [91] |
| PoHex (P. oxalicum) | GlcNAc (300) | pNP-GlcNAc (30) | 10:1 | GlcNAc-β-1,4-GlcNAc | 5.0 | 35 | 5.25 | 13 2 | [92] |
| GalNAc (500) | pNP-GalNAc (30 + 22) 6 | 17:1 | GalNAc-β-1,6-GalNAc | 5.0 | 35 | 6.25 | 34 2 | [92] | |
| PoHex (P. oxalicum CCF 2430) | GlcNAc (877), GalNAc (877) | pNP-GalNAc (175/292) | 5:1, 3:1 | GalNAc-β-1,4-GlcNAc (I), GalNAc-β-1,6-GlcNAc (II), GalNAc-β-1,6-GalNAc (III) | 4.5 | 37 | 3.5 | 26.5 (I), 19 (II), 87 (III) 2,7 | [87] |
| TfHex (T. flavus CCF 2686) | GlcNAc (350) | 6-OH-GlcNAc-β-O-pNP (50) | 7:1 | GlcANAc-β-1,4-GlcNAc | 5.0 | 37 | 4 | 23 2,8 | [63] |
| GlcNAc (300) | 6-SO3-GlcNAc-β-O-pNP (75) | 4:1 | 6-SO3-GlcNAc-β-1,4-GlcNAc | 5.0 | 35 | 6.5 | 33 2 | [63] | |
| GlcNAc (300) | 6-OH-GlcNAc-β-O-pNP (30) | 10:1 | GlcANAc-β-1,4-GlcNAc | 5.0 | 37 | 4.5 | 37 2,8 | [93] | |
| GlcNAc-β-1,4-ManNAc (30) | 6-OH-GalNAc-β-O-pNP (73) | 1:2 | GalANAc-β-1,4-GlcNAc-β-1,4-ManNAc (I), GalANAc-β-1,4-GlcNAc (II) | 5.0 | 35 | 5 | 35 (I), 39 (II) 2,8 | [88] | |
| TfHex (T. flavus CCF 2686) | GlcNac, GalNAc, Glc, Gal (300) | pNP-GlcNAc, pNP-GalNAc (50) | 6:1 | GlcNAc-β-1,4-GlcNAc (I), GalNAc-β-1,6-GalNAc (II), GlcNAc-β-1,1-Glc (III), GlcNAc-β-1,1-Gal (IV) | 5.0 | 35 | 5 | 24 (I), 31 (II), 14 (III), 10 (IV) | [94] |
| a) Y470F | GlcNAc-β-linker (150) | pNP-GalNAc (50 + 50) 6 | 3:1 | GalNAc-β-1,4-GlcNAc-β-linker | 5.0 | 35 | 8.5 | 51 2,9 | [95] |
| b) Y470H | GlcNAc-β-N3 (200) | pNP-GalNAc (50) | 4:1 | GalNAc-β-1,4-GlcNAc-β-N3, GlcNAc-β-1,4-GlcNAc-β-N3 | 5.0 | 45 | 6.5 | n.a. 4,10 | [96] |
| GlcNAc (200), GlcNAc-β-O-EtN3 (150), GlcNAc-β-N3 (200) | pNP-GalNAc (50) | 4:1, 3:1 | GalNAc-β-1,4-GlcNAc (I), GalNAc-β-1,4-GlcNAc-β-O-EtN3 (II), GalNAc-β-1,4-GlcNAc-β-N3 (III) | 5.0 | 45 | 4 | 58 (I), 48 (II), 35 (III) 2,10 | [97] | |
| MurNAc-β-O-Pr (100) | pNP-GalNAc (50) | 2:1 | GalNAc-β-1,6-MurNAc-β-O-Pr | 5.0 | 35 | 5 | 1 | [94] | |
| c) Y470N | GlcNAc-β-linker (50) | pNP-GlcNAc (50 + 25) 6 | 1:1 | (GlcNAc)2-β-linker (I), (GlcNAc)3-β-linker (II), (GlcNAc)4-β-linker (III) (all β-1,4) | 5.0 | 35 | 8 | 26.8 (I+II+III) 2 | [64] |
| GlcNAc-β-linker (75) | pNP-GlcNAc (50 + 50) 6 | 2:1 | (GlcNAc)2-β-linker (I), (GlcNAc)3-β-linker (II), (GlcNAc)4-β-linker (III), (GlcNAc)5-β-linker (IV) (all β-1,4) | 5.0 | 35 | 8 | 58 (I+II+III+IV) | [98] | |
| GlcNAc-β-O-EtN3 (100) | pNP-GlcNAc (50 + 50) 6 | 2:1 | (GlcNAc)2-β-O-EtN3 (I), (GlcNAc)3-β-O-EtN3 (II), (GlcNAc)4-β-O-EtN3 (III), (GlcNAc)5-β-O-EtN3 (IV), (GlcNAc)6-β-O-EtN3 (V) (all β-1,4) | 5.0 | 35 | 8 | 21.5 (I), 18 (II), 11.1 (III), 2.4 (IV), 3.4 (V) 2 | [99] | |
| ThHex (Trichoderma harzianum CCF 2687) | UDP-GlcNAc (32) | pNP-GalNAc (208) | 1:5 | GalNAc-β-1,4-GlcNAc-α-UDP | 6.5 | 30 | 8 | 22 2,11 | [100] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AuLnb (Aureobacterium sp. L-101) | Lac (182) | pNP-LNB (18) | 10:1 | LNT (β-1,3) | 5.5 | 40 | 5 | 3.7 2 | [69] |
| BbhI (B. bifidum JCM 1254) | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 1.5 | 46 3,7 | [101] |
| Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | 0.6 | 16 3 | [102] | |
| Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 1.5 | 45 2,7 | [103] | |
| Lac (400) | pNP-GalNAc (10) | 40:1 | GalNAc-β-1,3-Lac | 5.8 | 45 | 4 | 55 2,7 | [103] | |
| a) R577K | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | 0.6 | 36 3 | [102] |
| b) H603F | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | n.a. | n.a. 3 | [102] |
| c) D606N | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | n.a. | 9 3 | [102] |
| d) D746A | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 8 | 40 3,4,7 | [101] |
| Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 58 3,7 | [104] | |
| e) D746C/G/S/V | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 62–73 3,7 | [104] |
| f) D746E | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 1 | 71 3,7 | [101] |
| Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 63 3,7 | [104] | |
| g) D746I | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 46 3,7 | [104] |
| h) D746Q | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 6 | 3 3,4,7 | [101] |
| Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 0 | [104] | |
| i) D746T | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | 3 | 85 3,4,7 | [104] |
| j) D746F/H/K/L/M/N/P/R/W/Y | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 0 | [104] |
| k) W801H | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | n.a. | 17 3 | [102] |
| l) W805A/G/N/S/T/V | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 45–48 3,7 | [104] |
| m) W805C/I/K/L/P | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 60–69 3,7 | [104] |
| n) W805D/E/F/H/M/Q/Y | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | n.a. | 16–40 3,7 | [104] |
| o) W805R | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | 3 | 82 3,7 | [104] |
| p) Y827F | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 55 | 1 | 53 3,7 | [101] |
| q) W882H | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | 1.3 | 66 3 | [102] |
| r) D884N | Lac (40) | pNP-GlcNAc (10) | 4:1 | LNT II (β-1,3) | 7.0 | 37 | n.a. | 12 3 | [102] |
| s) RMe4 10 | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | 6 | 75 3,7 | [104] |
| t) RM34 11 | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | 6 | 69 3,7 | [104] |
| u) RMf125 12 | Lac (400) | pNP-GlcNAc (20) | 20:1 | LNT II (β-1,3) | 5.8 | 37 | 6 | 81 3,7 | [104] |
| LnbB (B. bifidum JCM 1254) | Lac (500) | pNP-LNB (5) | 100:1 | LNT (β-1,3) | 4.5 | 40 | n.a. | n.d. 3 | [51] |
| Lac (600) | pNP-LNB (20) | 30:1 | LNT (β-1,3) | 5.8 | 37 | 0.2 | 8 3,8 | [105] | |
| a) D320E | Lac (600) | pNP-LNB (20) | 30:1 | LNT (β-1,3) | 5.8 | 37 | 1 | 12 3,8 | [105] |
| b) D320A | Lac (600) | pNP-LNB (20) | 30:1 | LNT (β-1,3) | 5.8 | 37 | 27 | 10 3,8 | [105] |
| c) Y419F | Lac (600) | pNP-LNB (20) | 30:1 | LNT (β-1,3) | 5.8 | 37 | 9 | 13 3,8 | [105] |
| SpHexE314A (S. plicatus) | pNP-3/4/6-S-GlcNAc, pNP-3/4-S-GalNAc, pNP-4-S-ManNAc, pNP-4-S-Man (5 each) | pNP-GlcNAc, pNP-GalNAc (30 + 30) 5 | 1:6 | Different thioglycosides (β-S) 6 | 7 | 37 | < 1 | >99 3,9 | [24] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BtHex (Bos taurus) | 2-amino-6-benzyl-2-deoxy-Glc-β-S-Et (99/112) | pNP-GlcNAc (52), pNP-GalNAc (60) | 2:1 | GlcNAc-β-1,3-2-amino-6-benzyl-2-deoxy-Glc-β-S-Et (I), GalNAc-β-1,3-2-amino-6-benzyl-2-deoxy-Glc-β-S-Et (II) | 6.8, 6.3 | 30 | 50, 42 | 3.0 (I), 4.1 (II) 2 | [53] |
| CeHex (Canavalia ensiformis) | Man-α-O-Me (579), Gal-β-O-Me (579) | pNP-GlcNAc (62) | 9:1 | GlcNAc-β-1,3-Man-α-O-Me (I), GlcNAc-β-1,3-Gal-β-O-Me (II) | 6.5 | r.t. | 65 | 16.5 (I), 14.0 (II) 2,3 | [106] |
| CgHex (Chamelea gallina) | Gal-α-O-Me (515), Man-α-O-Me (515) | pNP-GlcNAc (50/100) | 10:1, 5:1 | GlcNAc-β-1,6-Man-α-O-Me (I), GlcNAc-β-1,6-Gal-α-O-Me (II) | 5.8 | r.t. | 65 | 8 (I), 5.6 (II) 2,3 | [73] |
| Gal-β-O-Me (515/644) | pNP-GlcNAc (50), pNP-GalNAc (73) | 10:1, 9:1, 26:1 | GlcNAc-β-1,3-Gal-β-O-Me (I), GlcNAc-β-1,6-Gal-β-O-Me (II), GalNAc-β-1,3-Gal-β-O-Me (III) | 8.5 | 37 | 80, 120 | 8.8 (I + II), 6.2 (III) 2 | [73] | |
| VrHex (Vigna radiata) | GlcNAc-β-N-Ac (138) | pNP-GlcNAc (14) | 10:1 | GlcNAc-β-1,6-GlcNAc-β-N-Ac | 6.5 | 37 | 8 | 12 2,4 | [49] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AoHex (A. oryzae) 2 | pNP-Glc (100), pNP-Gal (100) | GlcNAc-β-O-NPy (50) | 2:1 | GlcNAc-β-1,4-Glc-β-O-pNP (I), GlcNAc-β-1,4-Gal-β-O-pNP (II) | 6.0 | 25 | 0.75 | 8.6 (I), 6.1 (II) 3 | [72] |
| 4 | Glc, Gal, Man, 2-deoxy-Glc, GlcNAc, GlcNPr, GlcNFo (1100 each), maltose, Lac (570 each) | GlcNAc-β-O-Ph (33) | 33:1, 17:1 | GlcNAc-β-1,1/2/3/4/6-Glc (I), GlcNAc-β-1,?-Gal (II), GlcNAc-β-1,?-Man (III), GlcNAc-β-1,?-2-deoxy-Glc (IV), GlcNAc-β-1,?-GlcNAc (V), GlcNAc-β-1,?-GlcNPr (VI), GlcNAc-β-1,?-GlcNFo (VII), GlcNAc-β-1,?-maltose (VIII), GlcNAc-β-1,1-Lac (IX), autocondensation products | 4.5 | 37 | n.a. | 36 (I), 38 (II), 3 (III), 10 (IV), 18 (V), 11 (VI), 22 (VII), 24 (VIII), 31 (IX) 5 | [71] |
| CgHex (C. gallina) | Gal-α-O-Me (257) | GalNAc-α-O-oNP(10) | 26:1 | GlcNAc-α-1,3-Gal-α-O-Me | 5.8 | 37 | 120 | 6.3 3 | [73] |
| TfHex (T. flavus CCF 2686) | GlcNAc (306) | 4-deoxy-GlcNAc-β-O-Ph (75) | 4:1 | 4-deoxy-GlcNAc-β-1,4-GlcNAc (I), 4-deoxy-GlcNAc-β-1,6-GlcNAc (II), 4-deoxy-GlcNAc-β-1,6-4-deoxy-GlcNAc-β-O-Ph (III) | 5.0 | 35 | 5.5 | 7 (I), 6 (II), 14 (III) 3 | [65] |
| GalNAc-β-N3 (400) | GlcNAc-β-N3 (100) | 4:1 | GlcNAc-β-1,6-GalNAc-β-N3 | 5.0 | 35 | 3.5 | 22 3 | [66] |
3.1.3. N,N’-Diacetylchitobiose and Chitooligomers as Donor
3.1.4. Oxazoline-Derivates as Activated Donors
3.2. Acceptor:Donor Ratio
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Hex1 (metagenomic) | Lac (500) | (GlcNAc)2 (100) | 5:1 | LNT II (β-1,3) (I), other regioisomers (β-1,?) (II) | 8.0 | 25 | 2 | 2 (I), 0.1 (II) 2 | [109] |
| Lac (500) | (GlcNAc)2 (100) | 5:1 | LNT II (β-1,3) (I), other regioisomers (β-1,?) (II) | 7.0 | 25 | 8.5 | 5.7 (I + II) 2 | [117] | |
| a) GTDDA 8 | Lac (500) | (GlcNAc)2 (100) | 5:1 | LNT II (β-1,3) (I), other regioisomers (β-1,?) (II) | 7.0 | 25 | 24 | 23.3 (I + II) 2 | [117] |
| b) GTEPG 8 | Lac (500) | (GlcNAc)2 (100) | 5:1 | LNT II (β-1,3) (I), other regioisomers (β-1,?) (II) | 7.0 | 25 | 47 | 29.5 (I + II) 2 | [117] |
| Hex2 (metagenomic) | Lac (500) | (GlcNAc)2 (100) | 5:1 | LNT II (β-1,3) (I), other regioisomers (β-1,?) (II) | 6.0 | 25 | 2 | 8.3 (I), 9.1 (II) 2 | [109] |
| NoHex (N. orientalis IFO12806 3) | pNP-LacNAc (155) | (GlcNAc)2 (730) | 1:5 | GlcNAc-LacNAc-β-O-pNP (three regiosomers: β-1,3 and β-1,6) | 5.0 | 40 | 12 | 2.9–7.4 4,5 | [118] |
| Lac-β-O-Me (737) | (GlcNAc)2 (324) | 2:1 | LNT II-β-O-Me (β-1,3) (I), GlcNAc-β-1,6-Lac-β-O-Me (II), Gal-β-1,4-(GlcNAc-β-1,6)Glc-β-O-Me (III) | 5.0 | 40 | 20 | 17 (I + II + III) 4 | [119] | |
| pNP-Lac (168) | (GlcNAc)2 (317) | 1:2 | LNT II-β-O-pNP (β-1,3) (I), GlcNAc-β-1,6-Lac-β-O-pNP (II), Gal-β-1,4-(GlcNAc-β-1,6)Glc-β-O-pNP (III) | 5.0 | 40 | 12 | 2.4 (I + II + III) 4,6 | [119] | |
| Gal-β-1,3-GalNAc-α-O-pNP (23) | (GlcNAc)2 (239) | 1:10 | Gal-β-1,3-(GlcNAc-β-1,6-)GalNAc-α-O-pNP (I), GlcNAc-β-1,6-Gal-β-1,3-GalNAc-α-O-pNP (II), GlcNAc-β-1,3-Gal-β-1,3-GalNAc-α-O-pNP (III), Gal-β-1,3-(GlcNAc-β-1,6-)GalNAc-β-O-pNP (IV), GlcNAc-β-1,6-Gal-β-1,3-GalNAc-β-O-pNP (V), GlcNAc-β-1,3-Gal-β-1,3-GalNAc-β-O-pNP (VI), | 5.0 | 40 | 12 | 14 (I + II + III), 8 (IV + V + VI) 7 | [120] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D Ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BbhI (B. bifidum JCM1254) | Lac (600) | Glc-oxa (60) | 10:1 | LNT II (β-1,3) | 7.5 | 37 | 2 | 58 2 | [101] |
| a) D746E | Lac (600) | Glc-oxa (60) | 10:1 | LNT II (β-1,3) | 7.5 | 37 | 0.5 | 85 2 | [101] |
| Lac (600) | Glc-oxa (600) | 1:1 | LNT II (β-1,3) | 7.5 | 37 | 0.5 | 86 3 | [101] | |
| b) D746A | Lac (600) | Glc-oxa (60) | 10:1 | LNT II (β-1,3) | 7.5 | 37 | 5 | 80 2,4 | [101] |
| c) D746Q | Lac (600) | Glc-oxa (60) | 10:1 | LNT II (β-1,3) | 7.5 | 37 | 8 | 16 2,4 | [101] |
| d) Y827F | Lac (600) | Glc-oxa (60) | 10:1 | LNT II (β-1,3) | 7.5 | 37 | 2.5 | 80 2 | [101] |
| Hex1GTEPG 9 (metagenomic) | Lac (500) | Glc-oxa (100) | 5:1 | LNT II (I) (β-1,3), three unidentified regioisomers (β-1,?) (II) | 8.0 | 25 | 6.5 | >25 (I + II) 2 | [108] |
| LnbB (B. bifidum JCM 1254) | Lac (600) | Gal-β-1,3-Glc-oxa (LNB-oxa) (12) | 50:1 | LNT (β-1,3) | 7.5 | 37 | 0.1 | 67 2 | [105] |
| a) D320E | Lac (600) | LNB-oxa (12) | 50:1 | LNT (β-1,3) | 7.5 | 37 | 1 | 29 2,4 | [105] |
| b) D320A | Lac (600) | LNB-oxa (12) | 50:1 | LNT (β-1,3) | 7.5 | 37 | 21 | 13 2,4 | [105] |
| c) Y419F | Lac (600) | LNB-oxa (12) | 50:1 | LNT (β-1,3) | 7.5 | 37 | 2.5 | 37 2 | [105] |
| PhNah20A (Paraglaciecola hydrolytica S66T) | Lac (200) | Glc-oxa (100) | 2:1 | Lac-β-1,1-β-GlcNAc (I), LNT II (β-1,3) (II), unidentified regioisomer (β-1,?) (III) 7 | 8.0 | 37 | 2 | 3.8 (I + II + III) 2 | [111] |
| SpHex (S. plicatus) | Lac (200) | Glc-oxa (100) | 2:1 | LNT II (β-1,3), unidentified regioisomer (β-1,?) | 8.0 | 37 | 1 | n.a. 2,8 | [111] |
| a) E314A | 3/4/6-S-GlcNAc-β-O-pNP, 3/4-S-GalNAc-β-O-pNP, 4-S-ManNAc-β-O-pNP, 4-S-Man-β-O-pNP (5 each) | Glc-oxa (30) | 1:6 | Different thioglycosides (β-S) 5,6 | 7.0 | 37 | <1 | >99 2 | [24] |
| Enzyme (Organism) | Acceptor (c [mM]) | Donor (c [mM]) | A:D ratio | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| AnHex (A. niger CCIM K2) | t-Boc-Ser, t-Boc-Thr (1130) | α-GalNAc (1130) | 1:1 | GalNAc-α-O-Ser (I), GalNAc-α-O-Thr (II) | 4.8 | 35 | 168 | 7.4 (I), 3.6 (II) 2,3 | [23] | |
| AoHex (A. oryzae) 4 | Elymoclavine (95), Chanoclavine (95) | pNP-GalNAc (44) | 2:1 | GalNAc-β-O-elymoclavine (I), GalNAc-β-O-chanoclavine(II) | 4.5 | 33 | 60, 28 | 15 (I), 4.8 (II) 2,5 | [21] | |
| 4 | Ergometrine (95) | pNP-GalNAc (44) | 2:1 | GalNAc-β-O-ergometrine | 4.5 | 33 | 26 | 12 2 | [21] | |
| 4 | Elymoclavine (95), Chanoclavine (95) | pNP-GlcNAc (44) | 2:1 | GlcNAc-β-O-elymoclavine (I), GlcNAc-β-O-chanoclavine (II) | 4.5 | 28 | 70, 52 | 13.4 (I), 8.1 (II) 2 | [21] | |
| 4 | Ergometrine/Ergometrinine (95) | pNP-GlcNAc (44) | 2:1 | GlcNAc-β-O-ergometrine, GlcNAc-β-O-ergometrinine | 4.5 | 28 | 52 | 10.4 2 | [21] | |
| 6 | Sphingosine (1670) | GlcNAc (180), Et-GlcNAc (321), Pr-GlcNAc (683) 7 | 1:1 | GlcNAc-β-O-sphingosine (I), (GlcNAc)2 (β-1,4) (II), (GlcNAc)3 (β-1,4) (III) | 7.0 | 40 | 36 | 44 (I), 14 (II), 8 (III) 2 | [115,116] | |
| 4 | 1,3,5-tris(hydroxyethyl) cyanuric acid (Thca) (77), 1,4-benzenedimethanol (Bm) (145) | pNP-GlcNAc (58 + 2 × 29) 8 | 1:1, 3:1 | GlcNAc-β-O-Thca (I), GlcNAc-β-O-Bm (II) | 5.0 | n.a. | >120 | 29 (I), 12 (II) 2 | [19] | |
| 9 | Several alcohols, ethylene glycol, glycerol, erythritol, three alditols (910 each) | Ph-GlcNAc (1) | 910:1 | Alkyl-glycosides (β-O) (I) 10, autocondensation products | 4.5 | 37 | 0.6 | 27–86 (I) 11 | [71] | |
| AoHex (A. oryzae CCF 1066) | Thiamine (370) | pNP-GlcNAc (125 + 74 + 52) 8 | 3:1 | GlcNAc-β-O-thiamine | 5.0 | 37 | 11 | 3.5 2 | [22] | |
| Pyridoxine (195) | pNP-GlcNAc (93 + 4 × 9) 8 | 2:1 | GlcNAc-β-O-4a-pyridoxine (I), GlcNAc-β-O-5a-pyridoxine (II) | 5.0 | 24 | 3 | 13.5 (I + II) 2 | [121] | ||
| BbhI (B. bifidum JCM1254) | Different alcohols (20% (v/v) each) | LNT (1.3) | n.a. | n.d. | 6.0 | 40 | n.a. | n.a. 11 | [51] | |
| NoHex (Nocardia orientalis IFO12806 13) | 1,6-hexanediol (Hx) (50), triethylene glycol (Doo) (51) | (GlcNAc)4 (100) | 1:2 | GlcNAc-β-O-Hx (I)/Doo (II)-O-β-GlcNAc, GlcNAc-β-O-Hx (III)/Doo (IV), (GlcNAc)2-β-O-Hx (V)/Doo (VI) (β-1,4) | 6.7 | 40 | 45 | 10.5 (I), 22.6 (III), 1.4 (V); 4.7 (II), 69.0 (IV), 0.75 (VI) 2 | [20] | |
| PoHex (Penicillium oxalicum) | Cyclohexanol (Chx) (327), coniferyl alcohol (Con) (200) | pNP-GalNAc (30 + 2 × 15) 8 | 11:1, 7:1 | GalNAc-β-O-Chx (I), GalNAc-β-1,6-I (II), GalNAc-β-O-Con (III), GalNAc-β-1,6-III (IV) 14 | 5.0 | 35 | 7 | 21 (I), 5 (II), 20 (III), 3 (IV) 2 | [92] | |
| t-butanol | pNP-GalNAc (30) | n.a. | Not identified | 5.0 | 35 | 24 | 0.7 11 | [92] | ||
| Myo-inositol (500) | pNP-GalNAc (30 + 2 × 30) 8,19 | 17:1 | GalNAc-β-O-myo-inositol regioisomers I and II | 5.0 | 35 | 7 | 3 (I + II) 2 | [92] | ||
| Pyridine-3-aldoxime | pNP-GalNAc (30) 19 | n.a. | Not identified | 5.0 | 35 | 24 | <1 11 | [92] | ||
| PoHex (P. oxalicum IFO 5748) | Several alcohols (22% (v/v) each) | (GlcNAc)3 (16) | 236:1–88:1 | GlcNAc-β-O-alkyl | 4.5 | 37 | 1.5 | n.a. 12 | [122] | |
| 6-Benzyloxyhexan-1-ol (Bhx) (955) (20% (v/v)) | (GlcNAc)3 (16) | 60:1 | GlcNAc-β-O-Bhx | 4.5 | 37 | 1 | 32 2 | [122] | ||
| SmHex (Serratia marcescens YS-1) | Several alcohols, diols and one triol (8% (v/v) each) | (GlcNAc)2 (94) | 21:1–8:1 | (GlcNAc)3 (β-1,4) (I), GlcNAc-β-O-alkyl (II) | 6.0 | 40 | 24 | 15.3–65.9 (II), 2.2–26.7 (I) 11,15 | [62] | |
| Several alditols (8% (w/v) each) | (GlcNAc)2 (94) | 6:1–3:1 | (GlcNAc)3 (β-1,4) (I), GlcNAc-β-O-aldityl (II) 16 | 6.0 | 40 | 24 | 1.62–11.0 (II), 8.18–10.4 (I) 11 | [62] | ||
| SpHexE314A (Streptomyces plicatus) | 2,4-dinitrophenol (Dnp), pNP, phenol (20 each), 2-chlorophenol (Cp) (7) | Glc-oxa (5) 20 | 4:1, 1:1 | GlcNAc-β-O-Dnp/pNP/Ph/Cp 17 | 8.0 | 37 | <1 | >99 11 | [24] | |
| Et-Cys | pNP-GlcNAc | n.a. | GlcNAc-β-S-Cys 18 | n.a. | n.a. | n.a. | n.a. | [24] | ||
| Synuclein model peptides: VVCGV (0.002), AGCIA (0.002) | pNP-GlcNAc (10) 21 | 1:5000 | GlcNAc-β-S-peptide conjugates (VVCGV (I), AGCIA (II)) 18 | 7.0 | 37 | <1 | 44 (I), 30 (II) 11 | [24] | ||
| Tau protein (244-441) C301S C322S S400C (0.16) | pNP-GlcNAc (6.5) | 1:50 | GlcNAc-β-S-Tau conjugate 18 | 7.0 | 37 | 3 | 44 11 | [24] | ||
3.3. pH and Temperature Modifying Trans-Glycosylation Activity
3.4. Additives Increasing Trans-Glycosylation
3.4.1. Co-Solvents
3.4.2. Salts
3.4.3. Cyclodextrins
4. Increased Trans-Glycosylation Activity by Enzyme Engineering
4.1. Mutation of the Water-Stabilizing Tyr
4.2. Mutation of the Aglycone Binding Site
4.3. Mutation of the Catalytic Asp-Glu Pair
4.4. Mutation of Other Conserved Active Site Residues
4.5. A Non-Conserved Loop Close to the Active Site as Hotspot for Beneficial Mutation?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hackman, R.H.; Goldberg, M. Light-scattering and infrared-spectrophotometric studies of chitin and chitin derivatives. Carbohydr. Res. 1974, 38, 35–45. [Google Scholar] [CrossRef]
- Gooday, G. The Ecology of Chitin Degradation. In Advances in Microbial Ecology; Marshall, K., Ed.; Springer: Boston, MA, USA, 1990; pp. 387–430. [Google Scholar]
- Slámová, K.; Bojarová, P.; Petrásková, L.; Křen, V. β-N-Acetylhexosaminidase: What’s in a name...? Biotechnol. Adv. 2010, 28, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Duan, Y.; Yang, Q. Revisiting glycoside hydrolase family 20 β-N-acetyl-D-hexosaminidases: Crystal structures, physiological substrates and specific inhibitors. Biotechnol. Adv. 2018, 36, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J.; Schaub, Y.; Walther, A. N-acetylglucosamine utilization by Saccharomyces cerevisiae based on expression of Candida albicans NAG genes. Appl. Environ. Microbiol. 2009, 75, 5840–5845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faijes, M.; Castejón-Vilatersana, M.; Val-Cid, C.; Planas, A. Enzymatic and cell factory approaches to the production of human milk oligosaccharides. Biotechnol. Adv. 2019, 37, 667–697. [Google Scholar] [CrossRef]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of Human Milk Oligosaccharides: Protein Engineering Strategies for Improved Enzymatic Transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Bych, K.; Mikš, M.H.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs using microbial hosts—From cell engineering to large scale production. Curr. Opin. Biotechnol. 2019, 56, 130–137. [Google Scholar] [CrossRef]
- Frahn, J.; Edgar, J.; Jones, A.; Cockrum, P.; Anderton, N.; Culvenor, C. Structure of the corynetoxins, metabolites of Corynebacterium rathayi responsible for toxicity of annual ryegrass (Lolium rigidum) pastures. Aust. J. Chem. 1984, 37, 165–182. [Google Scholar] [CrossRef]
- Wright, D. The Orthosomycins, A New Family of Antibiotics. Tetrahedron 1979, 35, 1207–1237. [Google Scholar] [CrossRef]
- Ngo, D.-N.; Kim, M.-M.; Kim, S.-K. Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohydr. Polym. 2008, 74, 228–234. [Google Scholar] [CrossRef]
- Ngo, D.-N.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Production of chitin oligosaccharides with different molecular weights and their antioxidant effect in RAW 264.7 cells. J. Funct. Foods 2009, 1, 188–198. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Yang, S.; Niu, R.; Chu, E.; Lin, X. N-Acetylchitooligosaccharide is a potent angiogenic inhibitor both in vivo and in vitro. Biochem. Biophys. Res. Commun. 2007, 357, 26–31. [Google Scholar] [CrossRef]
- Latxague, L.; Gaubert, A.; Barthélémy, P. Recent Advances in the Chemistry of Glycoconjugate Amphiphiles. Molecules 2018, 23, 89. [Google Scholar] [CrossRef] [Green Version]
- Micheletto, Y.M.S.; da Silveira, N.P.; Barboza, D.M.; dos Santos, M.C.; de Lima, V.R.; Giacomelli, F.C.; Martinez, J.C.V.; Frizon, T.E.A.; Bó, A.G.D. Investigation of self-association between new glycosurfactant N-acetyl-β-D-glucosaminyl-PEG-docosanate and soybean phosphatidylcholine into vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 467, 166–172. [Google Scholar] [CrossRef]
- Kieburg, C.; Lindhorst, T.K.; Křen, V. Enzymatic Glycosylation of Branched Symmetrical Non-Carbohydrate Polyols. J. Carbohydr. Chem. 1998, 17, 1239–1247. [Google Scholar] [CrossRef]
- Misawa, Y.; Akimoto, T.; Amarume, S.; Murata, T.; Usui, T. Enzymatic synthesis of spacer-linked divalent glycosides carrying N-acetylglucosamine and N-acetyllactosamine: Analysis of cross-linking activities with WGA. J. Biochem. 2008, 143, 21–30. [Google Scholar] [CrossRef]
- Křen, V.; Ščigelová, M.; Přikrylová, V.; Havliček, V.; Sedmera, P. Enzymatic Synthesis of β-N-Acetylhexosaminides of Ergot Alkaloids. Biocatalysis 1994, 10, 181–193. [Google Scholar] [CrossRef]
- Křen, V.; Huňkova, Z.; Halada, P.; Suzuki, Y. Transglycosylation of thiamin by fungal β-N-acetylhexosaminidases. Biosci. Biotechnol. Biochem. 1998, 62, 2415–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weignerová, L.; Pelantová, H.; Manglová, D.; Michálková, K.; Křen, V. Condensation reactions catalyzed by α-N-acetylgalactosaminidase from Aspergillus niger yielding α-N-acetylgalactosaminides. Biocatal. Biotransform. 2010, 28, 150–155. [Google Scholar] [CrossRef]
- Tegl, G.; Hanson, J.; Chen, H.M.; Kwan, D.H.; Santana, A.G.; Withers, S.G. Facile Formation of β-thioGlcNAc Linkages to Thiol-Containing Sugars, Peptides, and Proteins using a Mutant GH20 Hexosaminidase. Angew. Chem. Int. Ed. Engl. 2019, 58, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Drouillard, S.; Armand, S.; Davies, G.J.; Vorgias, C.E.; Henrissat, B. Serratia marcescens chitobiase is a retaining glycosidase utilizing substrate Acetamido Group Participation. Biochem. J. 1997, 949, 945–949. [Google Scholar] [CrossRef] [Green Version]
- Mega, T.; Ikenaka, T.; Matsushima, Y. Studies on N-Acetyl-β-D-glucosaminidase of Aspergillus oryzae I. Purification and Characterization of N-Acetyl-β-D-glucosaminidase. J. Biochem. 1970, 68, 109–117. [Google Scholar]
- Jones, C.S.; Kosman, D.J. Purification, Properties, Kinetics, and Mechanism of β-N-Acetylglucosaminidase from Aspergillus niger. J. Biol. Chem. 1980, 255, 11861–11869. [Google Scholar]
- Knapp, S.; Vocadlo, D.; Gao, Z.; Kirk, B.; Lou, J.; Withers, S.G. NAG-thiazoline, An N-Acetyl-β-hexosaminidase Inhibitor That Implicates Acetamido Participation. J. Am. Chem. Soc. 1996, 118, 6804–6805. [Google Scholar] [CrossRef]
- Tews, I.; Perrakis, A.; Oppenheim, A.; Dauter, Z.; Wilson, K.S.; Vorgias, C.E. Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nat. Struct. Biol. 1996, 3, 638–648. [Google Scholar] [CrossRef]
- Koshland, D.E., Jr. Stereochemistry and the Mechanism of Enzymatic Reactions. Biol. Rev. 1953, 28, 416–436. [Google Scholar] [CrossRef]
- Teze, D.; Coines, J.; Raich, L.; Kalichuk, V.; Solleux, C.; Tellier, C.; André-Miral, C.; Svensson, B.; Rovira, C. A single point mutation converts GH84 O-GlcNAc hydrolases into phosphorylases. Experimental and theoretical evidence. J. Am. Chem. Soc. 2020, 142, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Coines, J.; Alfonso-Prieto, M.; Biarnés, X.; Planas, A.; Rovira, C. Oxazoline or Oxazolinium Ion? The Protonation State and Conformation of the Reaction Intermediate of Chitinase Enzymes Revisited. Chem. A Eur. J. 2018, 24, 19258–19265. [Google Scholar] [CrossRef] [PubMed]
- Umekawa, M.; Huang, W.; Li, B.; Fujita, K.; Ashida, H.; Wang, L.-X.; Yamamoto, K. Mutants of Mucor hiemalis Endo-β-N-acetylglucosaminidase Show Enhanced Transglycosylation and Glycosynthase-like Activities. J. Biol. Chem. 2008, 283, 4469–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissaro, B.; Monsan, P.; Fauré, R.; O’Donohue, M.J. Glycosynthesis in a waterworld: New insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 2015, 467, 17–35. [Google Scholar] [CrossRef]
- Bojarová, P.; Křen, V. Glycosidases: A key to tailored carbohydrates. Trends Biotechnol. 2009, 27, 199–209. [Google Scholar] [CrossRef]
- Bojarovà, P.; Křen, V. Glycosidases in carbohydrate synthesis: When organic chemistry falls short. CHIMIA Int. J. Chem. 2011, 65, 65–70. [Google Scholar] [CrossRef]
- Bojarová, P.; Bruthans, J.; Křen, V. β-N-Acetylhexosaminidases—The wizards of glycosylation. Appl. Microbiol. Biotechnol. 2019, 103, 7869–7881. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Withers, S.G. Detailed Comparative Analysis of the Catalytic Mechanisms of β-N-Acetylglucosaminidases from Families 3 and 20 of Glycoside Hydrolases. Biochemistry 2005, 44, 12809–12818. [Google Scholar] [CrossRef]
- Sakurama, H.; Kiyohara, M.; Wada, J.; Honda, Y.; Yamaguchi, M.; Fukiya, S.; Yokota, A.; Ashida, H.; Kumagai, H.; Kitaoka, M.; et al. Lacto-N-biosidase Encoded by a Novel Gene of Bifidobacterium longum Subspecies longum Shows Unique Substrate Specificity and Requires a Designated Chaperone for Its Active Expression. J. Biol. Chem. 2013, 288, 25194–25206. [Google Scholar] [CrossRef] [Green Version]
- Biosynth Carbosynth, UDP-GlcNAc Disodium Salt. Available online: https://www.carbosynth.com/carbosynth/website.nsf/(w-productdisplay)/218264098DDF79EC802573EE0073054C (accessed on 31 January 2020).
- Zeuner, B.; Jers, C.; Mikkelsen, J.D.; Meyer, A.S. Methods for improving enzymatic trans-glycosylation for synthesis of human milk oligosaccharide biomimetics. J. Agric. Food Chem. 2014, 62, 9615–9631. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzym. Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Ajisaka, K.; Nishida, H.; Fujimoto, H. The synthesis of oligosaccharides by the reversed hydrolysis reaction of β-glucosidase at high substrate concentration and at high temperature. Biotechnol. Lett. 1987, 9, 243–248. [Google Scholar] [CrossRef]
- Fujimoto, H.; Isomura, M.; Miyazaki, T.; Matsuo, I.; Walton, R.; Sakakibara, T.; Ajisaka, K. Enzymatic syntheses of GlcNAcβ1-2Man and Galβ1-4GlcNAcβ1-2Man as components of complex type sugar chains. Glycoconj. J. 1997, 14, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rajnochová, E.; Dvořáková, J.; Huňková, Z.; Křen, V. Reverse hydrolysis catalysed by β-N-acetylhexosaminidase from Aspergillus oryzae. Biotechnol. Lett. 1997, 19, 869–872. [Google Scholar] [CrossRef]
- Zhuravleva, N.V.; Luk’yanov, P.A.; Pivkin, M.V. N-Acetyl-β-D-hexosaminidase Secreted by the Marine Fungus Phoma glomerata. Appl. Biochem. Microbiol. 2004, 40, 448–453. [Google Scholar] [CrossRef]
- Mega, T.; Ikenaka, T.; Matsushima, Y. Studies on N-Acetyl-β-D-glucosaminidase of Aspergillus oryzae III. Transglycosylation by the Enzyme and Preparation of Crystalline Phenyl N, N′-Diacetyl-β-chitobioside. J. Biochem. 1972, 72, 1197–1203. [Google Scholar] [CrossRef]
- Lakshmanan, T.; Loganathan, D. Enzymatic synthesis of N-glycoprotein linkage region disaccharide mimetics using β-N-acetylhexosaminidases from Aspergillus oryzae and Vigna radiata. Tetrahedron Asymmetry 2005, 16, 255–260. [Google Scholar] [CrossRef]
- Matsuo, I.; Kim, S.; Yamamoto, Y.; Ajisaka, K.; Maruyama, J.I.; Nakajima, H.; Kitamoto, K. Cloning and overexpression of β-N-acetylglucosaminidase encoding gene nagA from Aspergillus oryzae and enzyme-catalyzed synthesis of human milk oligosaccharide. Biosci. Biotechnol. Biochem. 2003, 67, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Wada, J.; Ando, T.; Kiyohara, M.; Ashida, H.; Kitaoka, M.; Yamaguchi, M.; Kumagai, H.; Katayama, T.; Yamamoto, K. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl. Environ. Microbiol. 2008, 74, 3996–4004. [Google Scholar] [CrossRef] [Green Version]
- Rauvolfová, J.; Weignerová, L.; Kuzma, M.; Přikrylová, V.; Macková, M.; Pišvejcová, A.; Křen, V. Enzymatic synthesis of N-acetylglucosaminobioses by reverse hydrolysis: Characterisation and application of the library of fungal β-N-acetylhexosaminidases. J. Mol. Catal. B Enzym. 2004, 29, 259–264. [Google Scholar] [CrossRef]
- Nilsson, K.G.I.; Eliasson, A.; Pan, H.; Rohman, M. Synthesis of disaccharide derivatives employing β-N-acetyl-D-hexosaminidase, β-D-galactosidase and β-D-glucuronidase. Biotechnol. Lett. 1999, 21, 11–15. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, Y.; Ye, H.; Liu, J.; Uzawa, H. Synthesis of p-nitrophenyl sulfated disaccharides with β-D-(6-sulfo)-GlcNAc units using β-N-acetylhexosaminidase from Aspergillus oryzae in a transglycosylation reaction. Biotechnol. Lett. 2007, 29, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Packwood, J.; Samuel, C.J.; Critchley, P.; Crout, D.H.G. Glycosidase-catalysed oligosaccharide synthesis: Preparation of N-acetylchitooligosaccharides using the β-N-acetylhexosaminidase of Aspergillus oryzae. Carbohydr. Res. 1995, 279, 293–305. [Google Scholar] [CrossRef]
- Kubisch, J.; Weignerová, L.; Kötter, S.; Lindhorst, T.K.; Sedmera, P.; Křen, V. Enzymatic synthesis of p-nitrophenyl β-chitobioside. J. Carbohydr. Chem. 1999, 18, 975–984. [Google Scholar] [CrossRef]
- Singh, S.; Gallagher, R.; Derrick, P.J.; Crout, D.H.G. Glycosidase-catalysed oligosaccharide synthesis: Preparation of the N-acetylchitooligosaccharides penta-N-acetylchitopentaose and hexa-N-acetylchitohexaose using the β-N-acetylhexosaminidase of Aspergillus oryzae. Tetrahedron Asymmetry 1995, 6, 2803–2810. [Google Scholar] [CrossRef]
- Dvořáková, J.; Schmidt, D.; Huňková, Z.; Thiem, J.; Křen, V. Enzymatic rearrangement of chitine hydrolysates with β-N-acetylhexosaminidase from Aspergillus oryzae. J. Mol. Catal. B Enzym. 2001, 11, 225–232. [Google Scholar] [CrossRef]
- Tsujibo, H.; Kondo, N.; Tanaka, K.; Miyamoto, K.; Baba, N.; Inamori, Y. Molecular Analysis of the Gene Encoding a Novel Transglycosylative Enzyme from Alteromonas sp. Strain O-7 and Its Physiological Role in the Chitinolytic System. J. Bacteriol. 1999, 181, 5461–5466. [Google Scholar] [CrossRef] [Green Version]
- Nanjo, F.; Ishikawa, M.; Katsumi, R.; Sakai, K. Purification, Properties, and Transglycosylation Reaction of β-N-Acetylhexosaminidase from Nocardia orientalis. Agric. Biol. Chem. 1990, 54, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Fialová, P.; Weignerová, L.; Rauvolfová, J.; Přikrylová, V.; Pišvejcová, A.; Ettrich, R.; Kuzma, M.; Sedmera, P.; Křen, V. Hydrolytic and transglycosylation reactions of N-acyl modified substrates catalysed by β-N-acetylhexosaminidases. Tetrahedron 2004, 60, 693–701. [Google Scholar] [CrossRef]
- Kurakake, M.; Goto, T.; Ashiki, K.; Suenaga, Y.; Komaki, T. Synthesis of new glycosides by transglycosylation of N-acetylhexosaminidase from Serratia marcescens YS-1. J. Agric. Food Chem. 2003, 51, 1701–1705. [Google Scholar] [CrossRef]
- Bojarová, P.; Slámová, K.; Křenek, K.; Gažák, R.; Kulik, N.; Ettrich, R.; Pelantová, H.; Kuzma, M.; Riva, S.; Adámek, D.; et al. Charged hexosaminides as new substrates for β-N-acetylhexosaminidase-catalyzed synthesis of immunomodulatory disaccharides. Adv. Synth. Catal. 2011, 353, 2409–2420. [Google Scholar] [CrossRef]
- Slámová, K.; Krejzová, J.; Marhol, P.; Kalachova, L.; Kulik, N.; Pelantová, H.; Cvačka, J.; Křen, V. Synthesis of Derivatized Chitooligomers using Transglycosidases Engineered from the Fungal GH20 β-N-Acetylhexosaminidase. Adv. Synth. Catal. 2015, 357, 1941–1950. [Google Scholar] [CrossRef]
- Slámová, K.; Gažák, R.; Bojarová, P.; Kulik, N.; Ettrich, R.; Pelantová, H.; Sedmera, P.; Křen, V. 4-deoxy-substrates for β-N-acetylhexosaminidases: How to make use of their loose specificity. Glycobiology 2010, 20, 1002–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fialová, P.; Carmona, A.T.; Robina, I.; Ettrich, R.; Sedmera, P.; Přikrylová, V.; Petrásková-Hušáková, L.; Křen, V. Glycosyl azide—A novel substrate for enzymatic transglycosylations. Tetrahedron Lett. 2005, 46, 8715–8718. [Google Scholar] [CrossRef]
- Takahashi, M.; Mashiyama, T.; Suzuki, T. Purification and some characteristics of β-N-acetylglucosaminidase Produced by Vibrio sp. J. Ferment. Bioeng. 1993, 76, 356–360. [Google Scholar] [CrossRef]
- Biosynth Carbosynth, 4-Nitrophenyl 2-Acetamido-2-Deoxy-β-D-Glucopyranoside. Available online: https://www.carbosynth.com/carbosynth/website.nsf/(w-productdisplay)/1FFC21817D596F5C802570E5006E19C1 (accessed on 31 January 2020).
- Murata, T.; Inukai, T.; Suzuki, M.; Yamagishi, M.; Usui, T. Facile enzymatic conversion of lactose into lacto-N-tetraose and lacto-N-neotetraose. Glycoconj. J. 1999, 16, 189–195. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (PubChem Database), 4-Nitrophenol (CID = 980). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/980 (accessed on 31 January 2020).
- Mega, T.; Ikenaka, T.; Matsushima, Y. Studies on N-Acetyl-β-D-glucosaminidase of Aspergillus oryzae IV. Acceptor Specificity and Quantitative Representation of the Transglycosylation Reaction. J. Biochem. 1972, 72, 1391–1396. [Google Scholar] [CrossRef]
- Yasukochi, T.; Inaba, C.; Fukase, K.; Kusumoto, S. Nitropyridyl glycosides: New glycosyl donors for enzymatic transglycosylation. Tetrahedron Lett. 1999, 40, 6585–6589. [Google Scholar] [CrossRef]
- Nilsson, K.G.I. Enzymic synthesis of HexNAc-containing disaccharide glycosides. Carbohydr. Res. 1990, 204, 79–83. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (PubChem Database), 2-Hydroxy-3-Nitropyridine (CID = 22793). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Hydroxy-3-nitropyridine (accessed on 31 January 2020).
- National Center for Biotechnology Information (PubChem Database), 2-Nitrophenol (CID = 6947). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Nitrophenol (accessed on 31 January 2020).
- National Center for Biotechnology Information (PubChem Database), Phenol (CID = 996). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenol (accessed on 31 January 2020).
- National Center for Biotechnology Information (PubChem Database), Sodium Azide (CID = 33557). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-azide (accessed on 31 January 2020).
- Ogata, M.; Zeng, X.; Usui, T.; Uzawa, H. Substrate specificity of N-acetylhexosaminidase from Aspergillus oryzae to artificial glycosyl acceptors having various substituents at the reducing ends. Carbohydr. Res. 2007, 342, 23–30. [Google Scholar] [CrossRef]
- Uzawa, H.; Zeng, X.; Minoura, N. Synthesis of 6′-sulfodisaccharides by β-N-acetylhexosaminidase-catalyzed transglycosylation. Chem. Commun. 2003, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Crout, D.H.G.; Howarth, O.W.; Singh, S.; Swoboda, B.E.P.; Critchley, P.; Gibson, W.T. Biotransformations in carbohydrate synthesis. N-Acetylgalactosaminyl and N-acetylglucosaminyl transfer onto methyl α- and β-glucosides catalysed by the β-N-acetylhexosaminidase from Aspergillus oryzae. J. Chem. Soc. Chem. Commun. 1991, 1550–1551. [Google Scholar]
- Crout, D.H.G.; Singh, S.; Swoboda, B.E.P.; Critchley, P.; Gibson, W.T. Biotransformations in carbohydrates synthesis. N-Acetylgalactosaminyl transfer on to methyl N-acetyl-β-D-glucosaminide (methyl 2-acetamido-2-deoxy-β-D-glucopyranoside) and methyl N-acetyl-α-D-glucosaminide (methyl 2-acetamido-2-deoxy-α-D-glucopyranoside). J. Chem. Soc. Chem. Commun. 1992, 53, 704–705. [Google Scholar] [CrossRef]
- Singh, S.; Packwood, J.; Crout, D.H.G. Kinetic control of regioselectivity in glycosidase-catalysed disaccharide synthesis: Preparation of 2-acetamido-4-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-2-deoxy-D-glucopyranose (N,N′-diacetylchitobiose) and 2-acetamido-6-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-2-deoxy-D-glucopyranose. J. Chem. Soc. Chem. Commun. 1994, 2227–2228. [Google Scholar]
- Křen, V.; Rajnochová, E.; Huňková, Z.; Dvořáková, J.; Sedmera, P. Unusual nonreducing sugar GlcNAcβ(1↔1)Manβ formation by β-N-acetylhexosaminidase from Aspergillus oryzae. Tetrahedron Lett. 1998, 39, 9777–9780. [Google Scholar] [CrossRef]
- Singh, S.; Scigelova, M.; Vic, G.; Crout, D.H.G. Glycosidase-catalysed oligosaccharide synthesis of di-, tri- and tetra-saccharides using the N-acetylhexosaminidase from Aspergillus oryzae and the β-galactosidase from Bacillus circulans. J. Chem. Soc. Perkin Trans. 1 1996, 1921–1926. [Google Scholar] [CrossRef]
- Drozdová, A.; Bojarová, P.; Křenek, K.; Weignerová, L.; Henßen, B.; Elling, L.; Christensen, H.; Jensen, H.H.; Pelantová, H.; Kuzma, M.; et al. Enzymatic synthesis of dimeric glycomimetic ligands of NK cell activation receptors. Carbohydr. Res. 2011, 346, 1599–1609. [Google Scholar] [CrossRef]
- Hušáková, L.; Herkommerová-Rajnochová, E.; Semeňuk, T.; Kuzma, M.; Rauvolfová, J.; Přikrylová, V.; Ettrich, R.; Plíhal, O.; Bezouška, K.; Křen, V. Enzymatic Discrimination of 2-Acetamido-2-deoxy-D-mannopyranose-Containing Disaccharides Using β-N-Acetylhexosaminidases. Adv. Synth. Catal. 2003, 345, 735–742. [Google Scholar] [CrossRef]
- Weignerová, L.; Vavrušková, P.; Pišvejcová, A.; Thiem, J.; Křen, V. Fungal β-N-acetylhexosaminidases with high β-N-acetylgalactosaminidase activity and their use for synthesis of β-GalNAc-containing oligosaccharides. Carbohydr. Res. 2003, 338, 1003–1008. [Google Scholar] [CrossRef]
- Bojarová, P.; Křenek, K.; Kuzma, M.; Petrásková, L.; Bezouška, K.; Namdjou, D.J.; Elling, L.; Křen, V. N-Acetylhexosamine triad in one molecule: Chemoenzymatic introduction of 2-acetamido-2-deoxy-β-D-galactopyranosyluronic acid residue into a complex oligosaccharide. J. Mol. Catal. B Enzym. 2008, 50, 69–73. [Google Scholar] [CrossRef]
- Rauvolfová, J.; Kuzma, M.; Weignerová, L.; Fialová, P.; Přikrylová, V.; Pišvejcová, A.; Macková, M.; Křen, V. β-N-Acetylhexosaminidase-catalysed synthesis of non-reducing oligosaccharides. J. Mol. Catal. B Enzym. 2004, 29, 233–239. [Google Scholar] [CrossRef]
- Aboitiz, N.; Cañada, F.J.; Hušáková, L.; Kuzma, M.; Křen, V.; Jiménez-Barbero, J. Enzymatic synthesis of complex glycosaminotrioses and study of their molecular recognition by herein domains. Org. Biomol. Chem. 2004, 2, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Hušáková, L.; Riva, S.; Casali, M.; Nicotra, S.; Kuzma, M.; Huňková, Z.; Křen, V. Enzymatic glycosylation using 6-O-acylated sugar donors and acceptors: β-N-acetylhexosaminidase-catalysed synthesis of 6-O,N,N′-triacetylchitobiose and 6′-O,N,N′-triacetylchitobiose. Carbohydr. Res. 2001, 331, 143–148. [Google Scholar] [CrossRef]
- Nekvasilová, P.; Andreasová, I.; Petrásková, L.; Pelantová, H.; Křen, V.; Bojarová, P. A novel enzymatic tool for transferring GalNAc moiety onto challenging acceptors. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140319. [Google Scholar] [CrossRef] [PubMed]
- Fialová, P.; Namdjou, D.J.; Ettrich, R.; Přikrylová, V.; Rauvolfová, J.; Křenek, K.; Kuzma, M.; Elling, L.; Bezouška, K.; Křen, V. Combined application of galactose oxidase and β-N-acetylhexosaminidase in the synthesis of complex immunoactive N-acetyl-D-galactosaminides. Adv. Synth. Catal. 2005, 347, 997–1006. [Google Scholar] [CrossRef]
- Garcia-Oliva, C.; Hoyos, P.; Petrásková, L.; Kulik, N.; Pelantová, H.; Cabanillas, A.H.; Rumbero, Á.; Křen, V.; Hernáiz, M.J.; Bojarová, P. Acceptor Specificity of β-N-Acetylhexosaminidase from Talaromyces flavus: A Rational Explanation. Int. J. Mol. Sci. 2019, 20, 6181. [Google Scholar] [CrossRef] [Green Version]
- Bojarová, P.; Kulik, N.; Hovorková, M.; Slámová, K.; Pelantová, H.; Křen, V. The β-N-acetylhexosaminidase in the synthesis of bioactive glycans: Protein and reaction engineering. Molecules 2019, 24, 599. [Google Scholar] [CrossRef] [Green Version]
- Bojarová, P.; Kulik, N.; Slámová, K.; Hubálek, M.; Kotik, M.; Cvačka, J.; Pelantová, H.; Křen, V. Selective β-N-acetylhexosaminidase from Aspergillus versicolor—A tool for producing bioactive carbohydrates. Appl. Microbiol. Biotechnol. 2019, 103, 1737–1753. [Google Scholar] [CrossRef]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefał, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar]
- Laaf, D.; Bojarová, P.; Mikulová, B.; Pelantová, H.; Křen, V.; Elling, L. Two-Step Enzymatic Synthesis of β-D-N-Acetylgalactosamine-(1 → 4)-D-N-acetylglucosamine (LacdiNAc) Chitooligomers for Deciphering Galectin Binding Behavior. Adv. Synth. Catal. 2017, 359, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Bojarová, P.; Chytil, P.; Mikulová, B.; Bumba, L.; Konefał, R.; Pelantová, H.; Krejzová, J.; Slámová, K.; Petrásková, L.; Kotrchová, L.; et al. Glycan-decorated HPMA copolymers as high-affinity lectin ligands. Polym. Chem. 2017, 8, 2647–2658. [Google Scholar] [CrossRef] [Green Version]
- Nieder, V.; Kutzer, M.; Kren, V.; Gallego, R.G.; Kamerling, J.P.; Elling, L. Screening and characterization of β-N-acetylhexosaminidases for the synthesis of nucleotide-activated disaccharides. Enzym. Microb. Technol. 2004, 34, 407–414. [Google Scholar] [CrossRef]
- Schmölzer, K.; Weingarten, M.; Baldenius, K.; Nidetzky, B. Glycosynthase Principle Transformed into Biocatalytic Process Technology: Lacto-N-triose II Production with Engineered exo-Hexosaminidase. ACS Catal. 2019, 9, 5503–5514. [Google Scholar] [CrossRef]
- Teze, D.; Zhao, J.; Wiemann, M.; Ara, K.Z.G.; Lupo, R.; Rønne, M.; Carlström, G.; Duus, J.Ø.; Sanejouand, Y.-H.; O’Donohue, M.J.; et al. Rational Enzyme Design Without Structural Knowledge: A Sequence-Based Approach for Efficient Generation of Glycosylation Catalysts. ChemRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, L.; Jin, L.; Sun, B.; Gu, G.; Lu, L.; Xiao, M. Efficient and Regioselective Synthesis of β-GalNAc/GlcNAc-Lactose by a Bifunctional Transglycosylating β-N-Acetylhexosaminidase from Bifidobacterium bifidum. Appl. Environ. Microbiol. 2016, 82, 5642–5652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Jin, L.; Jiang, X.; Guo, L.; Gu, G.; Xu, L.; Lu, L.; Wang, F.; Xiao, M. Converting a β-N-acetylhexosaminidase into two trans-β-N-acetylhexosaminidases by domain-targeted mutagenesis. Appl. Microbiol. Biotechnol. 2020, 104, 661–673. [Google Scholar] [CrossRef]
- Schmölzer, K.; Weingarten, M.; Baldenius, K.; Nidetzky, B. Lacto-N-tetraose synthesis by wild-type and glycosynthase variants of the β-N-hexosaminidase from Bifidobacterium bifidum. Org. Biomol. Chem. 2019, 17, 5661–5665. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, K.G.I. Enzymic synthesis of di- and tri-saccharide glycosides, using glycosidases and β-D-galactoside 3-α-sialyl-transferase. Carbohydr. Res. 1989, 188, 9–17. [Google Scholar] [CrossRef]
- Biosynth Carbosynth, N,N’-Diacetylchitobiose. Available online: https://www.carbosynth.com/carbosynth/website.nsf/(w-productdisplay)/9D1175C2DBD500A680256A6B0035991A (accessed on 31 January 2020).
- Muschiol, J.; Meyer, A.S. A chemo-enzymatic approach for the synthesis of human milk oligosaccharide backbone structures. Z. Naturforsch. C J. Biosci. 2019, 74, 85–89. [Google Scholar] [CrossRef]
- Nyffenegger, C.; Nordvang, R.T.; Zeuner, B.; Łężyk, M.; Difilippo, E.; Logtenberg, M.J.; Schols, H.A.; Meyer, A.S.; Mikkelsen, J.D. Backbone structures in human milk oligosaccharides: Trans-glycosylation by metagenomic β-N-acetylhexosaminidases. Appl. Microbiol. Biotechnol. 2015, 99, 7997–8009. [Google Scholar] [CrossRef]
- White, T. 93. Studies in the amino-sugars. Part II. The action of dilute alkali solution on N-acylglucosamines. J. Chem. Soc. 1940, 428–437. [Google Scholar] [CrossRef]
- Visnapuu, T.; Teze, D.; Kjeldsen, C.; Lie, A.; Duus, J.Ø.; André-Miral, C.; Pedersen, L.H.; Stougaard, P.; Svensson, B. Identification and Characterization of a β-N-Acetylhexosaminidase with a Biosynthetic Activity from the Marine Bacterium Paraglaciecola hydrolytica S66T. Int. J. Mol. Sci. 2020, 21, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosynth Carbosynth, 2-Methyl-(1,2-dideoxy-a-D-glucopyrano)-[2,1-d]-2-Oxazoline. Available online: https://www.carbosynth.com/carbosynth/website.nsf/(w-productdisplay)/A52FF54BFDD9F3508025775B0041E6FF (accessed on 31 January 2020).
- Noguchi, M.; Tanaka, T.; Gyakushi, H.; Kobayashi, A.; Shoda, S.I. Efficient synthesis of sugar oxazolines from unprotected N-acetyl-2-amino sugars by using chloroformamidinium reagent in water. J. Org. Chem. 2009, 74, 2210–2212. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Fujieda, T.; Huang, W.C.; Ishihara, M.; Kobayashi, A.; Shoda, S.I. A practical one-step synthesis of 1,2-oxazoline derivatives from unprotected sugars and its application to chemoenzymatic β-N-acetylglucosaminidation of disialo-oligosaccharide. Helv. Chim. Acta 2012, 95, 1928–1936. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Monosaccharide-Alkyl Glycoside Glass Phases: Plasticization with Hydrophilic and Hydrophobic Molecules. Angew. Chem. Int. Ed. Engl. 2000, 39, 3801–3804. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Enzymatic Glycosylation in Plasticized Glass Phases: A Novel and Efficient Route to O-Glycosides. Angew. Chem. Int. Ed. Engl. 2000, 39, 3804–3808. [Google Scholar] [CrossRef]
- Jamek, S.B.; Muschiol, J.; Holck, J.; Zeuner, B.; Busk, P.K.; Mikkelsen, J.D.; Meyer, A.S. Loop Protein Engineering for Improved Transglycosylation Activity of a β-N-Acetylhexosaminidase. ChemBioChem 2018, 19, 1858–1865. [Google Scholar] [CrossRef]
- Murata, T.; Tashiro, A.; Itoh, T.; Usui, T. Enzymic synthesis of 3′-O- and 6′-O-N-acetylglucosaminyl-N-acetyllactosaminide glycosides catalyzed by β-N-acetyl-D-hexosaminidase from Nocardia orientalis. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 326–334. [Google Scholar] [CrossRef]
- Matahira, Y.; Tashiro, A.; Sato, T.; Kawagishi, H.; Usui, T. Enzymic synthesis of lacto-N-triose II and its positional analogues. Glycoconj. J. 1995, 12, 664–671. [Google Scholar] [CrossRef]
- Murata, T.; Itoh, T.; Usui, T. Enzymatic synthesis of β-D-Gal-(1- >3)-[β-D-GlcNAc- (1- >6)]-α-D-GalNAc-OC6H4NO2-p as a carbohydrate unit of mucin-type 2 core. Glycoconj. J. 1998, 15, 575–582. [Google Scholar] [CrossRef]
- Weignerová, L.; Suzuki, Y.; Huňková, Z.; Sedmera, P.; Havlíček, V.; Marek, R.; Křen, V. Pyridoxine as a Substrate for Screening Synthetic Potential of Glycosidases. Collect. Czechoslov. Chem. Commun. 1999, 64, 1325–1334. [Google Scholar] [CrossRef]
- Kadowaki, S.; Saskiawan, I.; Watanabe, J.; Yamamoto, K.; Bunno, M.; Ichihara, Y.; Kumagai, H. Transglycosylation activity of β-N-acetylhexosaminidase from Penicillium oxalicum and its application to synthesis of a drug carrier. J. Ferment. Bioeng. 1997, 83, 341–345. [Google Scholar] [CrossRef]
- Lundemo, P.; Karlsson, E.N.; Adlercreutz, P. Eliminating hydrolytic activity without affecting the transglycosylation of a GH1 β-glucosidase. Appl. Microbiol. Biotechnol. 2017, 101, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugaro, G.; Carrea, G.; Cremonesi, P.; Casellato, M.; Antonini, E. The Oxidation of Steroid Hormones by Fungal Laccase in Emulsion of Water and Organic Solvents. Arch. Biochem. Biophys. 1973, 159, 1–6. [Google Scholar] [CrossRef]
- Bourquelot, E.; Bridel, M. Action de l’emulsine sur la gentiopicrine, en milieu alcoolique. J. Pharm. Chim. 1911, 4, 385–390. [Google Scholar]
- Zaks, A.; Klibanov, A.M. Enzymatic Catalysis in Organic Media at 100 °C. Science 1984, 224, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.; Schapöhler, S.; Scheper, T.; Schügerl, K. Influences of reaction conditions on the enantioselective transesterification using Pseudomonas cepacia lipase. Tetrahedron Asymmetry 1991, 2, 1011–1014. [Google Scholar] [CrossRef]
- Cremonesi, P.; Casellato, M.M. Enzymic reactions in heterogeneous phase, preparation of 17α, 20β, 21-trihydroxypregn-4[14 C] en-3, 11-dione. J. Label. Compd. 1973, 9, 521–528. [Google Scholar] [CrossRef]
- Bridiau, N.; Issaoui, N.; Maugard, T. The effects of organic solvents on the efficiency and regioselectivity of N-acetyl-lactosamine synthesis, using the β-galactosidase from Bacillus circulans in hydro-organic media. Biotechnol. Prog. 2010, 26, 1278–1289. [Google Scholar] [CrossRef]
- Hansson, T.; Andersson, M.; Wehtje, E.; Adlercreutz, P. Influence of water activity on the competition between β-glycosidase-catalysed transglycosylation and hydrolysis in aqueous hexanol. Enzym. Microb. Technol. 2001, 29, 527–534. [Google Scholar] [CrossRef]
- Lang, M.; Kamrat, T.; Nidetzky, B. Influence of ionic liquid cosolvent on transgalactosylation reactions catalyzed by thermostable β-glycosylhydrolase CelB from Pyrococcus furiosus. Biotechnol. Bioeng. 2006, 95, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, B.; Riisager, A.; Mikkelsen, J.D.; Meyer, A.S. Improvement of trans-sialylation versus hydrolysis activity of an engineered sialidase from Trypanosoma rangeli by use of co-solvents. Biotechnol. Lett. 2014, 36, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Bayón, C.; Cortés, Á.; Berenguer, J.; Hernáiz, M.J. Highly efficient enzymatic synthesis of Galβ-(1 → 3)-GalNAc and Galβ-(1 → 3)-GlcNAc in ionic liquids. Tetrahedron 2013, 69, 4973–4978. [Google Scholar] [CrossRef] [Green Version]
- Kaftzik, N.; Wasserscheid, P.; Kragl, U. Use of Ionic Liquids to Increase the Yield and Enzyme Stability in the β-Galactosidase Catalysed Synthesis of N-Acetyllactosamine. Org. Process Res. Dev. 2002, 6, 553–557. [Google Scholar] [CrossRef]
- Giacomini, C.; Irazoqui, G.; Gonzalez, P.; Batista-Viera, F.; Brena, B.M. Enzymatic synthesis of galactosyl–xylose by Aspergillus oryzae β-galactosidase. J. Mol. Catal. B Enzym. 2002, 19–20, 159–165. [Google Scholar] [CrossRef]
- Bayón, C.; Cortés, Á.; Aires-Trapote, A.; Civera, C.; Hernáiz, M.J. Highly efficient and regioselective enzymatic synthesis of β-(1 → 3) galactosides in biosolvents. RSC Adv. 2013, 3, 12155–12163. [Google Scholar] [CrossRef] [Green Version]
- Slámová, K.; Bojarová, P. Engineered N-acetylhexosamine-active enzymes in glycoscience. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2070–2087. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]
- Škerlová, J.; Bláha, J.; Pachl, P.; Hofbauerová, K.; Kukačka, Z.; Man, P.; Pompach, P.; Novák, P.; Otwinowski, Z.; Brynda, J.; et al. Crystal structure of native β-N-acetylhexosaminidase isolated from Aspergillus oryzae sheds light onto its substrate specificity, high stability, and regulation by propeptide. FEBS J. 2018, 285, 580–598. [Google Scholar] [CrossRef] [Green Version]
- Straková, Z.; Slámová, K.; Kulik, N.; Křen, V. Transglycosidases engineered from the β-N-acetylhexosaminidase from Aspergillus oryzae (CBM13 Program Book, p. 149). Available online: https://cbm13.sciencesconf.org/data/pages/CBM13_Abstract_Book.pdf (accessed on 28 March 2020).
- Mackenzie, L.F.; Wang, Q.; Warren, R.A.J.; Withers, S.G. Glycosynthases: Mutant Glycosidases for Oligosaccharide Synthesis. J. Am. Chem. Soc. 1998, 120, 5583–5584. [Google Scholar] [CrossRef]
- Jahn, M.; Marles, J.; Warren, R.A.J.; Withers, S.G. Thioglycoligases: Mutant Glycosidases for Thioglycoside Synthesis. Angew. Chem. Int. Ed. Engl. 2003, 42, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Teze, D.; Hendrickx, J.; Czjzek, M.; Ropartz, D.; Sanejouand, Y.-H.; Tran, V.; Tellier, C.; Dion, M. Semi-rational approach for converting a GH1 β-glycosidase into a β-transglycosidase. Prot. Eng. Des. Sel. 2014, 27, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teze, D.; Daligault, F.; Ferrières, V.; Sanejouand, Y.H.; Tellier, C. Semi-rational approach for converting a GH36 α-glycosidase into an α-transglycosidase. Glycobiology 2015, 25, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissaro, B.; Durand, J.; Biarnés, X.; Planas, A.; Monsan, P.; O’Donohue, M.J.; Fauré, R. Molecular Design of Non-Leloir Furanose-Transferring Enzymes from an α-L-Arabinofuranosidase: A Rationale for the Engineering of Evolved Transglycosylases. ACS Catal. 2015, 5, 4598–4611. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Zhou, Y.; Li, J.; Gao, R.; Guo, Z. Engineering T. naphthophila β-glucosidase for enhanced synthesis of galactooligosaccharides by site-directed mutagenesis. Biochem. Eng. J. 2017, 127, 1–8. [Google Scholar] [CrossRef]
- Jers, C.; Michalak, M.; Larsen, D.M.; Kepp, K.P.; Li, H.; Guo, Y.; Kirpekar, F.; Meyer, A.S.; Mikkelsen, J.D. Rational Design of a New Trypanosoma rangeli Trans-Sialidase for Efficient Sialylation of Glycans. PLoS ONE 2014, 9, e83902. [Google Scholar] [CrossRef]







| Enzyme (Organism) | Substrate (c [mM]) | Product(s) | pH | T [°C] | t [h] | Yield [%] 1 | Ref. |
|---|---|---|---|---|---|---|---|
| AoHex (A. oryzae) | pNP-GlcNAc (34) | GlcNAc-β-1,3-GlcNAc-β-O-pNP | 7.0 | 50 | n.a. | 5.5 2 | [53] |
| 3 | pNP-GlcNAc (24) | GlcNAc-β-1,4-GlcNAc-β-O-pNP (I), GlcNAc- β-1,6-GlcNAc-β-O-pNP (II) | 6.0 | 35 | 6 | 8 (I), 1.5 (II) 2 | [54] |
| 3 | (GlcNAc)2 (864) | (GlcNAc)3 (I), (GlcNAc)4 (II) (all β-1,4) | 6.5 | 30 | 25 | 23.3 (I), 7.5 (II) 2 | [55] |
| 3 | pNP-GlcNAc (112) 16 | GlcNAc-β-1,4-GlcNAc-β-O-pNP (I), GlcNAc-β-1,6-GlcNAc-β-O-pNP (II) | 5.5 | 37 | 2.5 | 22.2 (I), 3.8 (II) 2,4 | [56] |
| 3 | (GlcNAc)4 (161), (GlcNAc)3 (122) | (GlcNAc)4 (I), (GlcNAc)5 (II), (GlcNAc)6 (III) (all β-1,4) | 6.5 | 30 | 54, 52 | 16 (II) 2,5, 9.2 (III) 2,5; 13.5 (I) 2,6, 12.7 (II) 2,6, 6.1 (III) 2,6 | [57] |
| 7 | GlcNAc-β-O-Ph (38) | GlcNAc-β-1,4-GlcNAc-β-O-Ph (I), GlcNAc-β-1,6-GlcNAc-β-O-Ph (II), GlcNAc-β-1,4-GlcNAc-β-1,4-GlcNAc-β-O-Ph (III) | 6.0 | 37 | 22 | 2.4 (I), 0.2 (II), 0.2 (III) 2 | [48] |
| 7 | 3-O-Me-GlcNAc-β-O-Ph, 6-O-Me-GlcNAc-β-O-Ph, GalNAc-β-O-Ph (each 33) | 3-O-Me-GlcNAc-β-1,4-3-O-Me-GlcNAc-β-O-Ph (I), 3-O-Me-GlcNAc-β-1,6-3-O-Me-GlcNAc-β-O-Ph (II), 6-O-Me-GlcNAc-β-1,4-6-O-Me-GlcNAc-β-O-Ph (III), GalNAc-β-1,?-GalNAc-β-O-Ph (IV) | 4.5 | 37 | 1, 5 | 12 (I + II), 42 (III), 7.9 (IV) 2 | [48] |
| AoHex (A. oryzae CCF 1066) | Mixture of GlcNAc and (GlcNAc)2–(GlcNAc)7 | Mixture enriched in (GlcNAc)2, (GlcNAc)6, (GlcNAc)7 and (GlcNAc)8 (all β-1,4) | 4.5 | 37 | 9 | n.a. | [58] |
| Hex99 (Alteromonas sp. O-7 20) | (GlcNAc)2 (24) | GlcNAc-β-1,6-GlcNAc | 7.5 | 50 | 0.5 | n.a. | [59] |
| NoHex (N. orientalis IFO12806 8) | (GlcNAc)2 (590) | GlcNAc-β-1,6-GlcNAc (I), (GlcNAc)3 (β-1,4) (II) | 5.0 | 30 | 40, 6 | 25 (I), 13 (II) 9 | [60] |
| PoHex (P. oxalicum CCF 2315) | pNP-GlcNPr (46)17 | GlcNPr-β-1,4-GlcNPr-β-O-pNP | 5.0 | 37 | 16 | 24 2 | [61] |
| SmHex (S. marcescens YS-1) | (GlcNAc)2 (94) 18 | (GlcNAc)3 (β-1,4) | 6.0 | 40 | 24 | 10.7 9,10, 14.3 9,11, 26.7 9,12 | [62] |
| TfHex (Talaromyces flavus CCF 2686) | pNP-GlcANAc (281) | GlcANAc-β-1,4-GlcANAc-β-O-pNP | 5.0 | 35 | 3.5 | 16 2 | [63] |
| 6-SO3-GlcANAc-β-O-pNP (100) | 6-SO3-GlcANAc-β-1,4-6-SO3-GlcANAc-β-O-pNP | 5.0 | 35 | 4 | 28 2 | [63] | |
| pNP-GlcNAc (50) | (GlcNAc)2, (GlcNAc)2-β-O-pNP, (GlcNAc)3-β-O-pNP (all β-1,4) | 5.0 | 35 | 6 | 12 2,13 | [64] | |
| 4-deoxy-GlcNAc-β-O-Ph (200) | 4-deoxy-GlcNAc-β-1,6-4-deoxy-GlcNAc-O-Ph | 5.0 | 35 | 2 | 14 2 | [65] | |
| pNP-GlcNFo (50) 17 | GlcNFo-β-1,4-GlcNFo-β-O-pNP | 5.0 | 37 | 3 | 16 2 | [61] | |
| pNP-GlcNGl (50) 19 | GlcNGl-β-1,4-GlcNGl-β-O-pNP | 5.0 | 37 | 1.7 | 78 2 | [61] | |
| GlcNAc-β-N3 (600) | GlcNAc-β-1,4-GlcNAc-β-N3 (I), GlcNAc-β-1,6-GlcNAc-β-N3 (II) | 5.0 | 35 | 7.5 | 32 (I), 16 (II) 2 | [66] | |
| a) Y470F | pNP-GlcNAc (50) | (GlcNAc)2-β-O-pNP, (GlcNAc)3-β-O-pNP, (GlcNAc)4-β-O-pNP (all β-1,4) | 5.0 | 35 | 6 | 41 2,14 | [64] |
| b) Y470H | pNP-GlcNAc (50) | (GlcNAc)2-β-O-pNP, (GlcNAc)3-β-O-pNP, (GlcNAc)4-β-O-pNP (all β-1,4) | 5.0 | 35 | 6 | 26 2,14 | [64] |
| c) Y470N | pNP-GlcNAc (50) | (GlcNAc)7-β-O-pNP and longer (all β-1,4) | 5.0 | 35 | 6 | n.a.14 | [64] |
| VsHex (Vibrio sp. P-6-1) | (GlcNAc)2, (GlcNAc)3, (GlcNAc)4 (2 each) | Unidentified oligosaccharides (all β-1,?) 15 | 6.5 | 40 | 0.5 | n.a.15 | [67] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muschiol, J.; Vuillemin, M.; Meyer, A.S.; Zeuner, B. β-N-Acetylhexosaminidases for Carbohydrate Synthesis via Trans-Glycosylation. Catalysts 2020, 10, 365. https://doi.org/10.3390/catal10040365
Muschiol J, Vuillemin M, Meyer AS, Zeuner B. β-N-Acetylhexosaminidases for Carbohydrate Synthesis via Trans-Glycosylation. Catalysts. 2020; 10(4):365. https://doi.org/10.3390/catal10040365
Chicago/Turabian StyleMuschiol, Jan, Marlene Vuillemin, Anne S. Meyer, and Birgitte Zeuner. 2020. "β-N-Acetylhexosaminidases for Carbohydrate Synthesis via Trans-Glycosylation" Catalysts 10, no. 4: 365. https://doi.org/10.3390/catal10040365