Sex and Gender Differences on the Impact of Metabolism-Disrupting Chemicals on Obesity: A Systematic Review
Abstract
:1. Introduction
1.1. Obesity
1.2. Metabolism-Disrupting Chemicals
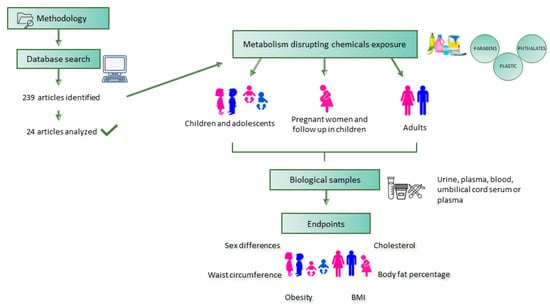
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Screening and Data Extraction
2.4. Study Quality Assessment
3. Results and Discussion
3.1. Studies on Children and Adolescents
3.2. Studies on Adults
3.3. Studies on Pregnant Women and Follow-Up in Children
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmanabhan, V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol. Metab. 2018, 29, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- COE. Gender Matters. Available online: https://www.coe.int/en/web/gender-matters/sex-and-gender (accessed on 10 December 2023).
- WHO. European Health Report 2018: More Than Numbers–Evidence for All; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Brandkvist, M.; Bjørngaard, J.H.; Ødegård, R.A.; Åsvold, B.O.; Sund, E.R.; Vie, G. Quantifying the impact of genes on body mass index during the obesity epidemic: Longitudinal findings from the HUNT Study. BMJ 2019, 366, l4067. [Google Scholar] [CrossRef] [PubMed]
- Močnik, M.; Marčun Varda, N. Obesogens in Children-An Uncharted Territory. Metabolites 2021, 11, 882. [Google Scholar] [CrossRef]
- Romacho, T.; Elsen, M.; Röhrborn, D.; Eckel, J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. The Impact of Obesity, Adipose Tissue, and Tumor Microenvironment on Macrophage Polarization and Metastasis. Biology 2022, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2020, 10, 615375. [Google Scholar] [CrossRef]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Sainsbury, A.; Group, S.C.W. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Strack, C.; Behrens, G.; Sag, S.; Mohr, M.; Zeller, J.; Lahmann, C.; Hubauer, U.; Loew, T.; Maier, L.; Fischer, M.; et al. Gender differences in cardiometabolic health and disease in a cross-sectional observational obesity study. Biol. Sex Differ. 2022, 13, 8. [Google Scholar] [CrossRef]
- Kapoor, N.; Arora, S.; Kalra, S. Gender Disparities in People Living with Obesity—An Unchartered Territory. J. Midlife Health 2021, 12, 103–107. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; D’Amore, A.; Giovannini, C.; Gessani, S.; Masella, R. Gender-related differences in lifestyle may affect health status. Ann. Ist. Super Sanita 2016, 52, 158–166. [Google Scholar] [CrossRef]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do nutritional behaviors depend on biological sex and cultural gender? Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Mohajer, N.; Du, C.Y.; Checkcinco, C.; Blumberg, B. Obesogens: How They Are Identified and Molecular Mechanisms Underlying Their Action. Front. Endocrinol. 2021, 12, 780888. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, P.; Fahmi, N.; Garg, B.; Dutta, S.; Sachar, S.; Matharu, A.S.; Vimaleswaran, K.S. Endocrine disruption and obesity: A current review on environmental obesogens. Curr. Res. Green Sustain. Chem. 2020, 3, 100009. [Google Scholar] [CrossRef]
- Henríquez-Hernández, L.A.; Luzardo, O.P.; Almeida-González, M.; Alvarez-León, E.E.; Serra-Majem, L.; Zumbado, M.; Boada, L.D. Background levels of polychlorinated biphenyls in the population of the Canary Islands (Spain). Environ. Res. 2011, 111, 10–16. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.M.; Huang, T.; Seely, E.W.; Saxena, A.R. The association between phthalates and metabolic syndrome: The National Health and Nutrition Examination Survey 2001–2010. Environ. Health 2016, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Küblbeck, J.; Vuorio, T.; Niskanen, J.; Fortino, V.; Braeuning, A.; Abass, K.; Rautio, A.; Hakkola, J.; Honkakoski, P.; Levonen, A.L. The EDCMET Project: Metabolic Effects of Endocrine Disruptors. Int. J. Mol. Sci. 2020, 21, 3021. [Google Scholar] [CrossRef] [PubMed]
- Tawar, N.; Banerjee, B.D.; Madhu, S.V.; Agrawal, V.; Gupta, S. Association of Organochlorine Pesticides with Genetic Markers of Endoplasmic Reticulum Stress in Type 2 Diabetes Mellitus: A Case-Control Study among the North-Indian Population. Front. Endocrinol. 2022, 13, 841463. [Google Scholar] [CrossRef]
- WHO/IPCS. Global Assessment on the State-of-the-Science of Endocrine Disruptors; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vom Saal, F.S.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health 2015, 14, 54. [Google Scholar] [CrossRef]
- Lind, L.; Lind, P.M.; Lejonklou, M.H.; Dunder, L.; Bergman, Å.; Guerrero-Bosagna, C.; Lampa, E.; Lee, H.K.; Legler, J.; Nadal, A.; et al. Uppsala Consensus Statement on Environmental Contaminants and the Global Obesity Epidemic. Environ. Health Perspect. 2016, 124, A81–A83. [Google Scholar] [CrossRef]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev. Endocr. Metab. Disord. 2007, 8, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Shahnazaryan, U.; Wójcik, M.; Bednarczuk, T.; Kuryłowicz, A. Role of Obesogens in the Pathogenesis of Obesity. Medicina 2019, 55, 515. [Google Scholar] [CrossRef] [PubMed]
- Decherf, S.; Seugnet, I.; Fini, J.B.; Clerget-Froidevaux, M.S.; Demeneix, B.A. Disruption of thyroid hormone-dependent hypothalamic set-points by environmental contaminants. Mol. Cell. Endocrinol. 2010, 323, 172–182. [Google Scholar] [CrossRef]
- Kirkley, A.G.; Sargis, R.M. Environmental endocrine disruption of energy metabolism and cardiovascular risk. Curr. Diab. Rep. 2014, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Temkin, A.M.; Bowers, R.R.; Ulmer, C.Z.; Penta, K.; Bowden, J.A.; Nyland, J.; Baatz, J.E.; Spyropoulos, D.D. Increased adiposity, inflammation, metabolic disruption and dyslipidemia in adult male offspring of DOSS treated C57BL/6 dams. Sci. Rep. 2019, 9, 1530. [Google Scholar] [CrossRef]
- Egusquiza, R.J.; Blumberg, B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology 2020, 161, bqaa024. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef]
- Janesick, A.; Blumberg, B. Obesogens, stem cells and the developmental programming of obesity. Int. J. Androl. 2012, 35, 437–448. [Google Scholar] [CrossRef]
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look forward. Front. Endocrinol. 2019, 10, 14. [Google Scholar] [CrossRef]
- OHAT. Risk of Bias Rating Tool for Human and Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2015. [Google Scholar]
- OHAT. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Toxicology Program: Research Triangle Park, NC, USA, 2019. [Google Scholar]
- Kim, J.; Chevrier, J. Exposure to parabens and prevalence of obesity and metabolic syndrome: An analysis of the Canadian Health Measures Survey. Sci. Total Environ. 2020, 713, 135116. [Google Scholar] [CrossRef]
- Yeung, E.H.; Bell, E.M.; Sundaram, R.; Ghassabian, A.; Ma, W.; Kannan, K.; Louis, G.M. Examining Endocrine Disruptors Measured in Newborn Dried Blood Spots and Early Childhood Growth in a Prospective Cohort. Obesity 2019, 27, 145–151. [Google Scholar] [CrossRef]
- Chen, M.; Lv, C.; Zhang, S.; Tse, L.A.; Hong, X.; Liu, X.; Ding, Y.; Xiao, P.; Tian, Y.; Gao, Y. Bisphenol A substitutes and childhood obesity at 7 years: A cross-sectional study in Shandong, China. Environ Sci. Pollut. Res. Int. 2023, 30, 73174–73184. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Woodward, M.; Bao, W.; Liu, B.; Trasande, L. Urinary Bisphenols and Obesity Prevalence among U.S. Children and Adolescents. J. Endocr. Soc. 2019, 3, 1715–1726. [Google Scholar] [CrossRef]
- Robles-Aguilera, V.; Gálvez-Ontiveros, Y.; Rodrigo, L.; Salcedo-Bellido, I.; Aguilera, M.; Zafra-Gómez, A.; Monteagudo, C.; Rivas, A. Factors Associated with Exposure to Dietary Bisphenols in Adolescents. Nutrients 2021, 13, 1553. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, D.; Li, Y.; Yang, Z.; Wang, X.; Chen, M.; Wang, Z.; Song, Y.; Zou, Z.; Ma, J. Effect of childhood phthalates exposure on the risk of overweight and obesity: A nested case-control study in China. Environ. Int. 2022, 158, 106886. [Google Scholar] [CrossRef]
- Vuong, A.M.; Braun, J.M.; Wang, Z.; Yolton, K.; Xie, C.; Sjodin, A.; Webster, G.M.; Lanphear, B.P.; Chen, A. Exposure to polybrominated diphenyl ethers (PBDEs) during childhood and adiposity measures at age 8 years. Environ. Int. 2019, 123, 148–155. [Google Scholar] [CrossRef]
- Vrijheid, M.; Fossati, S.; Maitre, L.; Márquez, S.; Roumeliotaki, T.; Agier, L.; Andrusaityte, S.; Cadiou, S.; Casas, M.; de Castro, M.; et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ. Health Perspect. 2020, 128, 67009. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.; Huh, D.A.; Moon, K.W. Urinary bisphenol concentrations and its association with metabolic disorders in the US and Korean populations. Environ. Pollut. 2022, 295, 118679. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, e114–e122. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Zhou, S.; Zhang, X.; Peng, C.; Zhou, H.; Tong, Y.; Lu, Q. Association of bisphenol A and its alternatives bisphenol S and F exposure with hypertension and blood pressure: A cross-sectional study in China. Environ. Pollut. 2020, 257, 113639. [Google Scholar] [CrossRef]
- Duan, Y.; Yao, Y.; Wang, B.; Han, L.; Wang, L.; Sun, H.; Chen, L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ. Pollut. 2018, 243, 1719–1726. [Google Scholar] [CrossRef]
- Shih, Y.L.; Hsieh, C.J.; Lee, T.Y.; Liao, P.H.; Wu, H.T.; Liu, C.Y. Sex Differences between Urinary Phthalate Metabolites and Metabolic Syndrome in Adults: A Cross-Sectional Taiwan Biobank Study. Int. J. Environ. Res. Public Health 2022, 19, 10458. [Google Scholar] [CrossRef]
- Sen, P.; Qadri, S.; Luukkonen, P.K.; Ragnarsdottir, O.; McGlinchey, A.; Jäntti, S.; Juuti, A.; Arola, J.; Schlezinger, J.J.; Webster, T.F.; et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 283–293. [Google Scholar] [CrossRef]
- Liu, G.; Dhana, K.; Furtado, J.D.; Rood, J.; Zong, G.; Liang, L.; Qi, L.; Bray, G.A.; DeJonge, L.; Coull, B.; et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018, 15, e1002502. [Google Scholar] [CrossRef]
- Lee, S.; Cho, S.R.; Jeong, I.; Park, J.B.; Shin, M.Y.; Kim, S.; Kim, J.H. Mercury Exposure and Associations with Hyperlipidemia and Elevated Liver Enzymes: A Nationwide Cross-Sectional Survey. Toxics 2020, 8, 47. [Google Scholar] [CrossRef]
- Akbar, L.; Zuk, A.M.; Martin, I.D.; Liberda, E.N.; Tsuji, L.J.S. Potential obesogenic effect of a complex contaminant mixture on Cree First Nations adults of Northern Québec, Canada. Environ. Res. 2021, 192, 110478. [Google Scholar] [CrossRef]
- Braun, J.M.; Li, N.; Arbuckle, T.E.; Dodds, L.; Massarelli, I.; Fraser, W.D.; Lanphear, B.P.; Muckle, G. Association between gestational urinary bisphenol a concentrations and adiposity in young children: The MIREC study. Environ. Res. 2019, 172, 454–461. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Wu, C.; Xiao, H.; Lv, S.; Lu, D.; Qi, X.; Feng, C.; Liang, W.; Chang, X.; et al. Urinary bisphenol A concentrations and adiposity measures at age 7 years in a prospective birth cohort. Chemosphere 2020, 251, 126340. [Google Scholar] [CrossRef]
- Güil-Oumrait, N.; Cano-Sancho, G.; Montazeri, P.; Stratakis, N.; Warembourg, C.; Lopez-Espinosa, M.J.; Vioque, J.; Santa-Marina, L.; Jimeno-Romero, A.; Ventura, R.; et al. Prenatal exposure to mixtures of phthalates and phenols and body mass index and blood pressure in Spanish preadolescents. Environ. Int. 2022, 169, 107527. [Google Scholar] [CrossRef]
- Højsager, F.D.; Kyhl, H.B.; Frederiksen, H.; Juul, A.; Andersson, A.M.; Andersen, M.S.; Grøntved, A.; Jensen, T.K. Prenatal Exposure to Butyl Paraben Is Associated with Fat Percentage in 7-Year-Old Boys. J. Clin. Endocrinol. Metab. 2021, 106, e2633–e2638. [Google Scholar] [CrossRef]
- Guo, J.; Miao, W.; Wu, C.; Zhang, J.; Qi, X.; Yu, H.; Chang, X.; Zhang, Y.; Zhou, Z. Umbilical cord serum PBDE concentrations and child adiposity measures at 7 years. Ecotoxicol. Environ. Saf. 2020, 203, 111009. [Google Scholar] [CrossRef]
- Kupsco, A.; Sjödin, A.; Cowell, W.; Jones, R.; Oberfield, S.; Wang, S.; Hoepner, L.A.; Gallagher, D.; Baccarelli, A.A.; Goldsmith, J.; et al. Prenatal exposure to polybrominated diphenyl ethers and BMI Z-scores from 5 to 14 years. Environ. Health 2022, 21, 82. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Fang, G.; Miao, M.; Liang, H.; Luan, M.; Liu, X.; Wen, S.; Chen, A.; Yuan, W. Association of prenatal exposure to polybrominated diphenyl ethers at low levels with adiposity measures in children up to 6 years. Chemosphere 2022, 303, 134867. [Google Scholar] [CrossRef]
- Tait, S.; Carli, F.; Busani, L.; Buzzigoli, E.; Della Latta, V.; Deodati, A.; Fabbrizi, E.; Gaggini, M.; Maranghi, F.; Tassinari, R.; et al. Biomonitoring of Bis(2-ethylhexyl)phthalate (DEHP) in Italian children and adolescents: Data from LIFE PERSUADED project. Environ. Res. 2020, 185, 109428. [Google Scholar] [CrossRef]
- Shah, B.; Tombeau Cost, K.; Fuller, A.; Birken, C.S.; Anderson, L.N. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutr. Prev. Health 2020, 3, 387–390. [Google Scholar] [CrossRef]
- Campbell, M.K. Biological, environmental, and social influences on childhood obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Roemmich, J.N.; Richmond, E.J.; Rogol, A.D.; Lovejoy, J.C.; Sheffield-Moore, M.; Mauras, N.; Bowers, C.Y. Endocrine control of body composition in infancy, childhood, and puberty. Endocr. Rev. 2005, 26, 114–146. [Google Scholar] [CrossRef]
- Varì, R.; Silenzi, A.; d’Amore, A.; Catena, A.; Masella, R.; Scazzocchio, B. MaestraNatura Reveals Its Effectiveness in Acquiring Nutritional Knowledge and Skills: Bridging the Gap between Girls and Boys from Primary School. Nutrients 2023, 15, 1357. [Google Scholar] [CrossRef]
- Wang, V.H.; Min, J.; Xue, H.; Du, S.; Xu, F.; Wang, H.; Wang, Y. What factors may contribute to sex differences in childhood obesity prevalence in China? Public Health Nutr. 2018, 21, 2056–2064. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Kur, P.; Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Wiszniewska, B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int. J. Environ. Res. Public Health 2020, 17, 2620. [Google Scholar] [CrossRef]
- González-Granillo, M.; Helguero, L.A.; Alves, E.; Archer, A.; Savva, C.; Pedrelli, M.; Ahmed, O.; Li, X.; Domingues, M.R.; Parini, P.; et al. Sex-specific lipid molecular signatures in obesity-associated metabolic dysfunctions revealed by lipidomic characterization in ob/ob mouse. Biol. Sex Differ. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.V.; Hall, J.M. Bisphenol A substitutes and obesity: A review of the epidemiology and pathophysiology. Front. Endocrinol. 2023, 14, 1155694. [Google Scholar] [CrossRef]
- Wen, Q.; Xie, X.; Zhao, C.; Ren, Q.; Zhang, X.; Wei, D.; Emanuelli, B.; Du, Y. The brominated flame retardant PBDE 99 promotes adipogenesis via regulating mitotic clonal expansion and PPARγ expression. Sci. Total Environ. 2019, 670, 67–77. [Google Scholar] [CrossRef]
- Davis, S.M.; Kaar, J.L.; Ringham, B.M.; Hockett, C.W.; Glueck, D.H.; Dabelea, D. Sex differences in infant body composition emerge in the first 5 months of life. J. Pediatr. Endocrinol. Metab. 2019, 32, 1235–1239. [Google Scholar] [CrossRef]
- Ortega-Avila, J.G.; García-Muñoz, H.; Segura Ordoñez, A.; Salazar Contreras, B.C. Sexual dimorphism of leptin and adiposity in children between 0 and 10 years: A systematic review and meta-analysis. Biol. Sex Differ. 2022, 13, 47. [Google Scholar] [CrossRef]



| Authors | Country | Age Range | N of Subjects | Chemicals | Biological Samples | Endpoints |
|---|---|---|---|---|---|---|
| Yeung et al. [47] | USA | 0–3 | 881 F; 969 M | Bisphenols | Blood | BMI, WFL |
| Chen et al. [48] | China | 7 | 200 F; 226 M | Bisphenols | Urine | BMI, waist circumference, body fat percentage |
| Liu et al. [49] | USA | 6–17 | 374 F; 371 M | Bisphenols | Urine | BMI, abdominal obesity |
| Jacobson et al. [50] | USA | 6–19 | 894 F; 937 M | Bisphenols | Urine | BMI, abdominal obesity |
| Robles-Aguilera et al. [51] | Spain | 12–16 | 273 F; 312 M | Bisphenols | Blood | BMI |
| Dong et al. [52] | China | 7–13 | 168 F; 519 M | Phthalates | Urine | BMI |
| Kim et al. [46] | Canada | 3–17 | 695 F; 723 M | Parabens | Urine | BMI, waist circumference, HDL cholesterol, triglycerides, fasting blood glucose and blood pressure |
| Vuong et al. [53] | USA | 1, 2, 3, 5 and 8 | 114 F; 92 M | Polybrominated diphenyl ethers | Blood | BMI, waist circumference and body fat percentage |
| Vrijheid et al. [54] | 6 European Countries | 6–11 | 711 M; 590 F | Organochlorine compounds, polybrominated diphenyl ethers, per- and polyfluoroalkyl substances, metals and elements, phthalate metabolites, phenols, organophosphates, pesticide metabolites, cotinine | Urine | BMI, waist circumference, skinfold thickness and body fat mass |
| Fan et al. [55] | USA | 6–19 | 2659 F; 2745 M | Heavy metals | Blood | BMI, triglyceride, cholesterol, low-density lipoprotein and HOMA-IR |
| Authors | Country | Age Range | N of Subjects | Chemicals | Biological Samples | Endpoints |
|---|---|---|---|---|---|---|
| Choi et al. [56] | USA; Republic of Korea | 20–70 | USA: 550 F; 496 M Republic of Korea: 1764 F; 1504 M | Bisphenols | Urine | BMI, HDL cholesterol and triglyceride levels |
| Liu et al. [57] | USA | ≥20 | 789 F; 732 M | Bisphenols | Urine | BMI and abdominal obesity |
| Jiang et al. [58] | China | 56 ± 9 | 582 F; 855 M | Bisphenols | Urine | Blood pressure |
| Duan et al. [59] | China | 51–58 | Controls: 156 F; 95 M Cases: 106 F; 145 M | Bisphenols | Urine | Type 2 Diabetes Mellitus |
| Shih et al. [60] | Taiwan | 30–70 | 693 F; 644 M | Phthalates | Urine | Waist circumference, blood pressure, fasting blood glucose, fasting serum triglycerides, high-density lipid cholesterol |
| Sen et al. [61] | USA | 18–75 | 70 F; 35 M | Perfluorooctanesulfonate compounds, perfluorohexanesulfonic acid, polyfluorinated compounds | Plasma | Liver fat content, HOMA-IR |
| Liu et al. [62] | USA | 30–70 | 384 F; 237 M | Perfluorooctanesulfonic acid, polyfluorinated compounds, perfluorohexanesulfonic acid, perfluorodecanoic acid | Plasma | Body weight and RMR |
| Kim et al. [46] | Canada | >18 | 568 F; 568 M | Parabens | Urine | BMI, waist circumference, HDL cholesterol, triglycerides, fasting blood glucose and blood pressure |
| Lee et al. [63] | Republic of Korea | 19–80 | 3687 F; 2767 M | Mercury | Blood and Urine | Total cholesterol, HDL, and triglycerides, hepatic enzymes (ALT, AST, GGT) |
| Akbar et al. [64] | Canada | 20–65 | 415 F; 280 M | 10 persistent organic pollutants and toxic metals | Plasma | BMI, waist circumference and body fat percentage |
| Authors | Country | Age Range | N of Subjects | Chemicals | Biological Samples | Endpoints in Children |
|---|---|---|---|---|---|---|
| Braun et al. [65] | Canada | Mothers: 18–35 Children: 1.9–6.2 | Mothers: 719 Children: 363 F; 356 M | Bisphenols | Urine | BMI, waist circumference and subscapular skinfold thickness |
| Guo et al. [66] | China | Mothers: 17 to ≥35 Children: 3 and 7 | Mothers: 363 Children: 157 F; 206 M | Bisphenols | Urine | BMI, waist circumference and skinfold thickness |
| Güil-Oumrait et al. [67] | Spain | Mothers: 31 Children: 11 | Mothers: 1015 Children: 500 F; 515 M | Phthalates and phenols | Urine | BMI, systolic and diastolic blood pressure |
| Højsager et al. [68] | Denmark | Mothers: 15–49 Children: 7 | Mothers: 312 Children: 156 F; 156 M | Parabens | Urine | BMI, fat percentage, android and gynoid fat percentage |
| Guo et al. [69] | China | Mothers:17–35 Children: 7 | Mothers: 318 Children: 138 F; 180 M | Polybrominated diphenyl ethers | Umbilical cord serum | BMI, waist circumference and skinfold thickness |
| Kupsco et al. [70] | USA | Mothers: 25 Children: 5–11 | Mothers: 260 Children: 145 F; 115 M | Polybrominated diphenyl ethers | Umbilical cord blood and peripheral blood | BMI |
| Chen et al. [71] | China | Mothers: <25–≥35 Children: 0–6 | Mothers: 340 Children: 148 F; 192 M | Polybrominated diphenyl ethers | Umbilical cord blood | BMI, arm circumference and waist circumference |
| Subjects | Contaminants | Obesity Outcomes | Sex | References |
|---|---|---|---|---|
| Children | BPA, BPS, BPF | Abdominal obesity and overweight | M | Liu et al. [49] Jacobson et al. [50] |
| Children | BPAF | Obesity and overweight | M | Chen et al. [48] |
| Children | Phtalathes | obesity | M | Dong et al. [52] |
| Children | PFOS, PFOA | Adiposity parameters | M | Yeung et al. [47] |
| Children | Mercury/selenium | Cholesterol level | F | Fan et al. [55] |
| Children | Zinc | Cholesterol level | M | Fan et al. [55] |
| Adults | MEP, BPA, BPS, parabens | Obesity | M | Kim et al. [46], Liu et al. [57], Jiang et al. [58], Shih et al. [60] |
| Adults | BPF | BMI and HDL-C levels | F | Choi et al. [56] |
| Adults | BPA | HDL-C levels | M | Choi et al. [56] |
| Pregnant women and follow-up in children | BPA | Adiposity parameters, obesity | F | Braun et al. [65], Guo et al. [66] |
| Pregnant women and follow-up in children | BP3 | BMI | F | Guil-Oumrait et al. [67] |
| Pregnant women and follow-up in children | Parabens | Adiposity parameters | M | Højsager et al. [68] |
| Pregnant women and follow-up in children | BDE-154, BDE-153, BDE-100 BDE-28 | Adiposity parameters | M | Guo et al. [69] |
| Pregnant women and follow-up in children | BDE-153, BDE-100 | Adiposity parameters | F | Guo et al. [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Archivio, M.; Coppola, L.; Masella, R.; Tammaro, A.; La Rocca, C. Sex and Gender Differences on the Impact of Metabolism-Disrupting Chemicals on Obesity: A Systematic Review. Nutrients 2024, 16, 181. https://doi.org/10.3390/nu16020181
D’Archivio M, Coppola L, Masella R, Tammaro A, La Rocca C. Sex and Gender Differences on the Impact of Metabolism-Disrupting Chemicals on Obesity: A Systematic Review. Nutrients. 2024; 16(2):181. https://doi.org/10.3390/nu16020181
Chicago/Turabian StyleD’Archivio, Massimo, Lucia Coppola, Roberta Masella, Alessia Tammaro, and Cinzia La Rocca. 2024. "Sex and Gender Differences on the Impact of Metabolism-Disrupting Chemicals on Obesity: A Systematic Review" Nutrients 16, no. 2: 181. https://doi.org/10.3390/nu16020181