Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review
Abstract
:1. Introduction
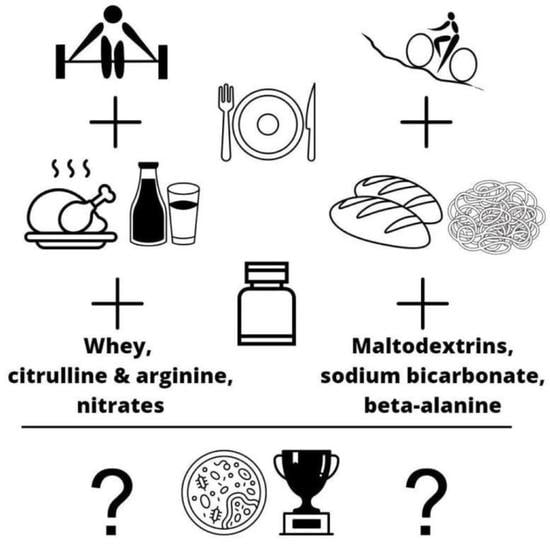
2. Aerobic Exercise—Diet and Supplements
3. Anaerobic Exercise—Diet and Supplements
4. Prebiotics for Athletes
5. Probiotics for Athletes
6. Ergogenics and Gut Microbiota—Animal Studies
7. Prebiotics, Probiotics and Gut Microbiota—Animal Studies
8. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quercia, S.; Candela, M.; Giuliani, C.; Turroni, S.; Luiselli, D.; Rampelli, S.; Brigidi, P.; Franceschi, C.; Bacalini, M.G.; Garagnani, P.; et al. From lifetime to evolution: Timescales of human gut microbiota adaptation. Front. Microbiol. 2014, 5, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, K.F.; Sayols-Baixeras, S.; Baldanzi, G.; Nowak, C.; Hammar, U.; Nguyen, D.; Varotsis, G.; Brunkwall, L.; Nielsen, N.; Eklund, A.C.; et al. An online atlas of human plasma metabolite signatures of gut microbiome composition. Nat. Commun. 2022, 13, 5370. [Google Scholar] [CrossRef]
- O’Donovan, C.M.; Madigan, S.M.; Garcia-Perez, I.; Rankin, A.; Sullivan, O.O.; Cotter, P. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J. Sci. Med. Sport 2020, 23, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [Green Version]
- Tooley, K. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Albillos, A.; De Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.W.; Agirman, G.; Hsiao, E.Y. The Gut Microbiome as a Regulator of the Neuroimmune Landscape. Annu. Rev. Immunol. 2022, 40, 143–167. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.P.; Poeze, M.; Van Loon, L.J.C.; DeJong, C.H.C.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef] [Green Version]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.S.; Lee, H.-B.; Kim, Y.; Park, H.-Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiącek, J.; Szurkowska, J.; Kryściak, J.; Galecka, M.; Karolkiewicz, J. No changes in the abundance of selected fecal bacteria during increased carbohydrates consumption period associated with the racing season in amateur road cyclists. PeerJ 2023, 11, e14594. [Google Scholar] [CrossRef] [PubMed]
- Olbricht, H.; Twadell, K.; Sandel, B.; Stephens, C.; Whittall, J.B. Is There a Universal Endurance Microbiota? Microorganisms 2022, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Kulecka, M.; Fraczek, B.; Mikula, M.; Zeber-Lubecka, N.; Karczmarski, J.; Paziewska, A.; Ambrozkiewicz, F.; Jagusztyn-Krynicka, K.; Cieszczyk, P.; Ostrowski, J. The composition and richness of the gut microbiota differentiate the top Polish endurance athletes from sedentary controls. Gut Microbes 2020, 11, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Mata, F.; Valenzuela, P.L.; Gimenez, J.; Tur, C.; Ferreria, D.; Domínguez, R.; Sanchez-Oliver, A.J.; Sanz, J.M.M. Carbohydrate Availability and Physical Performance: Physiological Overview and Practical Recommendations. Nutrients 2019, 11, 1084. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.R.; Chassaing, B. Maltodextrin, Modern Stressor of the Intestinal Environment. Cell. Mol. Gastroenterol. Hepatol. 2017, 7, 475–476. [Google Scholar] [CrossRef] [Green Version]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Ferreira, A.M.J.; Farias-Junior, L.F.; A A Mota, T.; Elsangedy, H.M.; Marcadenti, A.; Lemos, T.M.A.M.; Okano, A.H.; Fayh, A.P.T. Carbohydrate Mouth Rinse and Hydration Strategies on Cycling Performance in 30 Km Time Trial: A Randomized, Crossover, Controlled Trial. J. Sports Sci. Med. 2018, 17, 181–187. [Google Scholar]
- Hadzic, M.; Eckstein, M.L.; Schugardt, M. The Impact of Sodium Bicarbonate on Performance in Response to Exercise Duration in Athletes: A Systematic Review. J. Sports Sci. Med. 2019, 18, 271–281. [Google Scholar]
- Wilson, P. Sport Supplements and the Athlete’s Gut: A Review. Int. J. Sports Med. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Gravina, A.G.; Romeo, M.; Pellegrino, R.; Tuccillo, C.; Federico, A.; Loguercio, C. Just Drink a Glass of Water? Effects of Bicarbonate–Sulfate–Calcium–Magnesium Water on the Gut–Liver Axis. Front. Pharmacol. 2022, 13, 869446. [Google Scholar] [CrossRef]
- Murakami, S.; Goto, Y.; Ito, K.; Hayasaka, S.; Kurihara, S.; Soga, T.; Tomita, M.; Fukuda, S. The Consumption of Bicarbonate-Rich Mineral Water Improves Glycemic Control. Evidence-Based Complement. Altern. Med. 2015, 2015, 824395. [Google Scholar] [CrossRef] [Green Version]
- Jukić, I.; Kolobarić, N.; Stupin, A.; Matić, A.; Kozina, N.; Mihaljević, Z.; Mihalj, M.; Šušnjara, P.; Stupin, M.; Ćurić, Ž.B.; et al. Carnosine, Small but Mighty—Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef]
- Grgic, J. Effects of beta-alanine supplementation on Yo–Yo test performance: A meta-analysis. Clin. Nutr. ESPEN 2021, 43, 158–162. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.D. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef]
- Szurkowska, J.; Wiącek, J.; Laparidis, K.; Karolkiewicz, J. A Comparative Study of Selected Gut Bacteria Abundance and Fecal pH in Bodybuilders Eating High-Protein Diet and More Sedentary Controls. Nutrients 2021, 13, 4093. [Google Scholar] [CrossRef]
- Daher, J.; Mallick, M.; El Khoury, D. Prevalence of Dietary Supplement Use among Athletes Worldwide: A Scoping Review. Nutrients 2022, 14, 4109. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Lassen, P.B.; Belda, E.; Prifti, E.; Dao, M.C.; Specque, F.; Henegar, C.; Rinaldi, L.; Wang, X.; Kennedy, S.P.; Zucker, J.-D.; et al. Protein supplementation during an energy-restricted diet induces visceral fat loss and gut microbiota amino acid metabolism activation: A randomized trial. Sci. Rep. 2021, 11, 15620. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tian, L.; Hong, H.; Wang, Q.; Zhan, X.; Luo, Y.; Tan, Y. In Vitro Gut Fermentation of Whey Protein Hydrolysate: An Evaluation of Its Potential Modulation on Infant Gut Microbiome. Nutrients 2022, 14, 1374. [Google Scholar] [CrossRef]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaafsma, A.; Mallee, L.; Belt, M.V.D.; Floris, E.; Kortman, G.; Veldman, J.; Ende, D.V.D.; Kardinaal, A. The Effect of a Whey-Protein and Galacto-Oligosaccharides Based Product on Parameters of Sleep Quality, Stress, and Gut Microbiota in Apparently Healthy Adults with Moderate Sleep Disturbances: A Randomized Controlled Cross-Over Study. Nutrients 2021, 13, 2204. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Soy bioactive peptides and the gut microbiota modulation. Appl. Microbiol. Biotechnol. 2020, 104, 9009–9017. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef]
- Gough, L.A.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F.; Newbury, J.W.; Trexler, E.; Faghy, M.A.; Bridge, C.A. A critical review of citrulline malate supplementation and exercise performance. Eur. J. Appl. Physiol. 2021, 121, 3283–3295. [Google Scholar] [CrossRef]
- Van Wijck, K.; Wijnands, K.A.P.; Meesters, D.; Boonen, B.; Van Loon, L.J.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K.; Poeze, M. L-Citrulline Improves Splanchnic Perfusion and Reduces Gut Injury during Exercise. Med. Sci. Sports Exerc. 2014, 46, 2039–2046. [Google Scholar] [CrossRef]
- Curis, E.; Crenn, P.; Cynober, L. Citrulline and the gut. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 620–626. [Google Scholar] [CrossRef]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, Z.; Liu, M.; Chen, L.; Ding, W.; Zhang, H. Amino Acids Regulate Glycolipid Metabolism and Alter Intestinal Microbial Composition. Curr. Protein Pept. Sci. 2020, 21, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; He, J.; Wang, L.; Zhou, S.; Peng, X.; Huang, S.; Zheng, L.; Cheng, L.; Hao, Y.; Li, J.; et al. Ecological Effect of Arginine on Oral Microbiota. Sci. Rep. 2017, 7, 7206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, B.S.; Laranjinha, J. Nitrate from diet might fuel gut microbiota metabolism: Minding the gap between redox signaling and inter-kingdom communication. Free. Radic. Biol. Med. 2020, 149, 37–43. [Google Scholar] [CrossRef] [PubMed]
- González-Soltero, R.; Bailén, M.; De Lucas, B.; Ramírez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef]
- Leclerc, M.; Bedu-Ferrari, C.; Etienne-Mesmin, L.; Mariadassou, M.; Lebreuilly, L.; Tran, S.-L.; Brazeau, L.; Mayeur, C.; Delmas, J.; Rué, O.; et al. Nitric Oxide Impacts Human Gut Microbiota Diversity and Functionalities. mSystems 2021, 6, e0055821. [Google Scholar] [CrossRef]
- Bai, Y.; Gilbert, R.G. Mechanistic Understanding of the Effects of Pectin on In Vivo Starch Digestion: A Review. Nutrients 2022, 14, 5107. [Google Scholar] [CrossRef]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef]
- Sutehall, S.; Muniz-Pardos, B.; Bosch, A.; Pitsiladis, Y. The Effect of Sodium Alginate and Pectin Added to a Carbohydrate Beverage on Endurance Performance, Substrate Oxidation and Blood Glucose Concentration: A Systematic Review and Meta-analysis. Sports Med. Open 2022, 8, 82. [Google Scholar] [CrossRef]
- Flood, T.; Montanari, S.; Wicks, M.; Blanchard, J.; Sharpe, H.; Taylor, L.; Kuennen, M.R.; Lee, B.J. Addition of pectin-alginate to a carbohydrate beverage does not maintain gastrointestinal barrier function during exercise in hot-humid conditions better than carbohydrate ingestion alone. Appl. Physiol. Nutr. Metab. 2020, 45, 1145–1155. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Ponder, M.A.; McMillan, R.P.; Hughes, M.D.; Hulver, M.W.; Neilson, A.P.; Davy, K.P. Prebiotic Inulin Supplementation and Peripheral Insulin Sensitivity in adults at Elevated Risk for Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients 2021, 13, 3235. [Google Scholar] [CrossRef]
- Williams, C.J.; Torquati, L.; Li, Z.; A Lea, R.; Croci, I.; Keating, E.; Little, J.P.; Eynon, N.; Coombes, J.S. Oligofructose-Enriched Inulin Intake, Gut Microbiome Characteristics, and the V˙O2 Peak Response to High-Intensity Interval Training in Healthy Inactive Adults. J. Nutr. 2021, 152, 680–689. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, X.; Luo, Y.; Chen, B.; Ma, D.; Zhu, J. Effect of Fructooligosaccharides Supplementation on the Gut Microbiota in Human: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3298. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Li, X.; Zheng, F.; Zeng, X. Yeast β-glucan, a potential prebiotic, showed a similar probiotic activity to inulin. Food Funct. 2020, 11, 10386–10396. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Lin, K.; Guo, S.; Hou, Y.; Ma, R.; Wang, Q.; Wang, R. Plasma Metabolomics Reveals β-Glucan Improves Muscle Strength and Exercise Capacity in Athletes. Metabolites 2022, 12, 988. [Google Scholar] [CrossRef]
- Zabriskie, H.A.; Blumkaitis, J.C.; Moon, J.M.; Currier, B.S.; Stefan, R.; Ratliff, K.; Harty, P.S.; Stecker, R.A.; Rudnicka, K.; Jäger, R.; et al. Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise. Nutrients 2020, 12, 1144. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.K. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, S.; Zhang, Y.; Yang, J.; Hu, Z. Gut microbiota and calcium balance. Front. Microbiol. 2022, 13, 1033933. [Google Scholar] [CrossRef]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santibañez-Gutierrez, A.; Fernández-Landa, J.; Calleja-González, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachex Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Di Dio, M.; Calella, P.; Cerullo, G.; Pelullo, C.P.; Di Onofrio, V.; Gallè, F.; Liguori, G. Effects of Probiotics Supplementation on Risk and Severity of Infections in Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11534. [Google Scholar] [CrossRef]
- Guo, Y.-T.; Peng, Y.-C.; Yen, H.-Y.; Wu, J.-C.; Hou, W.-H. Effects of Probiotic Supplementation on Immune and Inflammatory Markers in Athletes: A Meta-Analysis of Randomized Clinical Trials. Medicina 2022, 58, 1188. [Google Scholar] [CrossRef]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, L.; Zhang, X.; Zhang, X.; Zhang, Y.; Zhang, Y.; Zheng, K.; Zheng, K.; Xiang, Q.; Xiang, Q.; et al. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Brencher, L.; Verhaegh, R.; Kirsch, M. Attenuation of intestinal ischemia-reperfusion-injury by β-alanine: A potentially glycine-receptor mediated effect. J. Surg. Res. 2017, 211, 233–241. [Google Scholar] [CrossRef]
- Silva, M.T.B.; Palheta-Junior, R.C.; Sousa, D.F.; Fonseca-Magalhães, P.A.; Okoba, W.; Campos, C.P.S.; Oliveira, R.B.; Magalhães, P.J.C.; Santos, A.A. Sodium bicarbonate treatment prevents gastric emptying delay caused by acute exercise in awake rats. J. Appl. Physiol. 2014, 116, 1133–1141. [Google Scholar] [CrossRef] [Green Version]
- Boscaini, S.; Cabrera-Rubio, R.; Nychyk, O.; Speakman, J.R.; Cryan, J.F.; Cotter, P.D.; Nilaweera, K.N. Age- and duration-dependent effects of whey protein on high-fat diet-induced changes in body weight, lipid metabolism, and gut microbiota in mice. Physiol. Rep. 2020, 8, e14523. [Google Scholar] [CrossRef]
- Tranberg, B.; Hellgren, L.I.; Lykkesfeldt, J.; Sejrsen, K.; Jeamet, A.; Rune, I.; Ellekilde, M.; Nielsen, D.S.; Hansen, A.K. Whey Protein Reduces Early Life Weight Gain in Mice Fed a High-Fat Diet. PLoS ONE 2013, 8, e71439. [Google Scholar] [CrossRef] [Green Version]
- Boscaini, S.; Cabrera-Rubio, R.; Golubeva, A.; Nychyk, O.; Fülling, C.; Speakman, J.R.; Cotter, P.D.; Cryan, J.F.; Nilaweera, K.N. Depletion of the gut microbiota differentially affects the impact of whey protein on high-fat diet-induced obesity and intestinal permeability. Physiol. Rep. 2021, 9, e14867. [Google Scholar] [CrossRef]
- Świątecka, D.; Złotkowska, D.; Markiewicz, L.H.; Szyc, A.M.; Wróblewska, B. Impact of whey proteins on the systemic and local intestinal level of mice with diet induced obesity. Food Funct. 2017, 8, 1708–1717. [Google Scholar] [CrossRef]
- Sprong, R.; Schonewille, A.; Van Der Meer, R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium–induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef]
- Osowska, S.; Moinard, C.; Loï, C.; Neveux, N.; Cynober, L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 2004, 53, 1781–1786. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Chen, S.; Yin, J.; Duan, J.; Li, T.; Liu, G.; Feng, Z.; Tan, B.; Yin, Y.; Wu, G. Dietary Arginine Supplementation of Mice Alters the Microbial Population and Activates Intestinal Innate Immunity. J. Nutr. 2014, 144, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Viana, M.L.; Santos, R.G.; Generoso, S.V.; Arantes, R.M.; Correia, M.I.T.; Cardoso, V.N. Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition 2010, 26, 218–223. [Google Scholar] [CrossRef]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Jimenez, B.; Lodge, S.; et al. How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2020, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Scholte, J.; Borewicz, K.; Bogert, B.V.D.; Smidt, H.; Scheurink, A.J.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, Y.; Lin, D.; Zheng, P.; Hu, M.; Wang, Q.; Pan, W.; Yang, X.; Hu, T.; Li, Q.; et al. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, C.; Cheng, Y. β-Glucan alleviates mice with ulcerative colitis through interactions between gut microbes and amino acids metabolism. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Lee, M.-C.; Chen, M.-J.; Huang, H.-W.; Wu, W.-K.; Lee, Y.-W.; Kuo, H.-C.; Huang, C.-C. Probiotic Lactiplantibacillus plantarum Tana Isolated from an International Weightlifter Enhances Exercise Performance and Promotes Antifatigue Effects in Mice. Nutrients 2022, 14, 3308. [Google Scholar] [CrossRef]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Sung, H.-C.; Huang, C.-C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Lin, K.-J.; Hsu, H.-Y.; Chiou, S.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Lactobacillus plantarum TWK10 Attenuates Aging-Associated Muscle Weakness, Bone Loss, and Cognitive Impairment by Modulating the Gut Microbiome in Mice. Front. Nutr. 2021, 8, 708096. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Zhang, Y.; Wang, S.; Li, F. Effect of Lactobacillus plantarum KSFY01 on the exercise capacity of D-galactose-induced oxidative stress-aged mice. Front. Microbiol. 2022, 13, 1030833. [Google Scholar] [CrossRef]
- Ducray, H.A.G.; Globa, L.; Pustovyy, O.; Roberts, M.D.; Rudisill, M.; Vodyanoy, V.; Sorokulova, I. Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3. J. Appl. Microbiol. 2020, 128, 1163–1178. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces boulardii Increases the Maximal Oxygen Consumption and Maximal Aerobic Speed Attained by Rats Subjected to an Incremental-Speed Exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef] [Green Version]
| Sport Supplement | Effective | Possibly Effective | Possibly Harmful | Not Known |
|---|---|---|---|---|
| Aerobic exercise | ||||
| Maltodextrin/glucose | X | |||
| Sodium bicarbonate | X | |||
| Beta-alanine | X | |||
| Anaerobic exercise | ||||
| Protein isolates | X | |||
| Citrulline and arginine | X | |||
| Nitrates | X | |||
| Studied Product | Effective | Possibly Effective | Possibly Harmful | Not Known |
|---|---|---|---|---|
| Prebiotics | ||||
| Inulin/FOS | X | |||
| Pectin/alginate | X | |||
| Beta-glucan | X | |||
| Probiotics | ||||
| Lactobacillus spp. | X | |||
| Bifidobacterium spp. | X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiącek, J.; Karolkiewicz, J. Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review. Nutrients 2023, 15, 1541. https://doi.org/10.3390/nu15061541
Wiącek J, Karolkiewicz J. Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review. Nutrients. 2023; 15(6):1541. https://doi.org/10.3390/nu15061541
Chicago/Turabian StyleWiącek, Jakub, and Joanna Karolkiewicz. 2023. "Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review" Nutrients 15, no. 6: 1541. https://doi.org/10.3390/nu15061541