A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia
Abstract
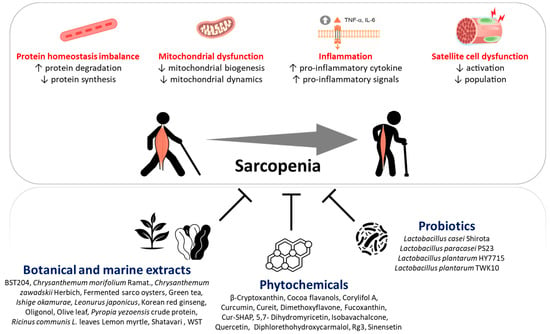
:1. Introduction
2. Sarcopenia Pathophysiology
2.1. Protein Homeostasis Imbalance
2.2. Age-Related Chronic Low-Grade Inflammation
2.3. Mitochondrial Dysfunction
2.3.1. Reduction of Mitochondrial Biogenesis
2.3.2. Dysregulation of Mitochondrial Dynamics
2.4. Satellite Cell Dysfunction
3. Beneficial Effects of Natural Dietary Ingredients on Skeletal Muscle
3.1. Improvement in Maintenance of Protein Homeostasis by Natural Dietary Ingredients
3.2. Protection against Inflammation by Natural Dietary Ingredients
| Natural Dietary Ingredients | Experimental Model | Experimental Designs | Results 1 (by Natural Dietary Ingredient Treatment) | Related Mechanisms (Potential Pathway) | Ref. |
|---|---|---|---|---|---|
| Botanical and marine extracts (bioactive compound) | |||||
| Non-saponin faction of Korean red ginseng | C57BL/6J mice | - young mice (3–6-month-old) - aged mice (20–24-month-old) - aged mice with ginseng (for 6 weeks) | ↓ CRP, IL-1, IL-6, TNF-α ↑ GSH ↑ Catalase, GPx, Sod1 | ↓ inflammation ↓ oxidative stress | [103] |
| Olive leaf extract | Wistar rats | - young rats (3-month-old) - aged rats (24-month-old) - aged rats with olive leaf extract (for 3 weeks) | ↓ COX-2, IL-1β, IL-6 ↑ IL-10 ↑ GPx | ↓ inflammation ↓ oxidative stress | [104] |
| Leonurus japonicus extract (leonurine) | L6 myotube | - Non-treatment - TNF-α and L. japonicus extract or leonurine | ↓ IL-6, TNF-α ↓ NF-κB | ↓ inflammation (NF-κB pathway) | [93] |
| Pyropia yezoensis crude protein | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and pyropia yezoensis protein | ↓ IL-6 ↓ TNF-R1, NF-κB, p-IκBα ↓ intracellular ROS level | ↓ inflammation (NF-κB pathway) ↓ oxidative stress | [106] |
| Phytochemicals | |||||
| Cur-SHAP | C2C12 myotube | - Non-treatment - LPS - LPS and Cur-SHAP | ↓ IL-6, TNF-α ↓ ROS generation | ↓ inflammation ↓ oxidative stress | [105] |
| Isobavachalcone | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and isobavachalcone | ↓ nuclear p-NF-κB p65 ↑ cytosolic p-NF-κB p65 ↑ HO-1, nuclear Nrf2 ↓ cytosolic Nrf2 | ↓ inflammation (NF-κB pathway) | [96] |
| Quercetin | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and quercetin | ↓ IκB-α | ↓ inflammation (NF-κB pathway) | [107] |
| Diphlorethohydroxycarmalol | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and diphlorethohydroxycarmalol | ↓ NO production ↓ IL-1β, IL-6, TNF-α ↓ p-IκB-α, p-NF-κB p65 | ↓ inflammation (NF-κB pathway) | [108] |
| Probiotics | |||||
| Lactobacillus casei Shirota | SAMP8 mice | - young mice (16-week-old) - aged mice (28-week-old) - aged mice with L. casei Shirota (for 12 weeks) | ↓ TNF-α ↑ IL-10 ↓ protein carbonyl content | ↓ inflammation ↓ oxidative stress | [109] |
| Lactobacillus paracasei PS23 | SAMP8 mice | - young mice (16-week-old) - aged mice (28-week-old) - aged mice with L. paracasei PS23 (for 12 weeks) | ↓ IL-6, MCP-1, TNF-α ↑ IL-10 ↓ protein carbonyl content ↑ GPx, Sod | ↓ inflammation ↓ oxidative stress | [110] |
3.3. Enhancement of Mitochondrial Function by Natural Dietary Ingredients
| Natural Dietary Ingredients | Experimental Model | Experimental Designs | Results 1 (by Natural Dietary Ingredient Treatment) | Related Mechanism (Potential Pathway) | Ref. |
|---|---|---|---|---|---|
| Botanical and marine extracts (bioactive compound) | |||||
| BST204 | C2C12 myotube | - Non-treatment - TNF-α (or Dex) - TNF-α (or Dex) and BST204 | ↑ Nrf1, Pgc-1α, Tfam ↑ Pgc-1α activity ↑ mitochondrial ATP content, MMP | ↑ mitochondrial biogenesis | [111,112] |
| Catechin-enriched green tea extract | C57BL/6JRj mice | - young mice (4-month-old) - aged mice (22-month-old) - aged mice with GTE (for 6 month) | ↑ Pgc-1α ↑ ATP5A | ↑ mitochondrial content | [114] |
| Oligonol | SAMP8 mice | - young mice (SAMR1) - aged mice (32-week-old) - aged mice with oligonol (for 8 weeks) | ↑ Ndufs8, Pgc-1α, Tfam ↑ mtDNA/nuclear DNA ratio ↑ Fis1, Mff, Mfn2, Opa1, PINK1 ↓ mitochondrial morphological changes (aberrance, swelling) | ↑ mitochondrial biogenesis ↑ mitochondrial dynamics | [95] |
| Ricinus communis L. leaves extract (rutin) | C2C12 myotube | - Non-treatment - Dex - Dex and R. communis L. leaves extract or rutin | ↑ mitochondrial OCR | ↑ mitochondrial respiratory capacity | [116] |
| Chrysanthemum zawadskii Herbich extract (Acacetin-7-O-β-D-rutinoside) | C2C12 myotube | - Non-treatment - Dex - Dex and C. zawadskii Herbich extract or Aca-ce-tin-7-O-β-D-rutinoside | ↑ mitochondrial OCR | ↑ mitochondrial respiratory capacity | [117] |
| Phytochemicals | |||||
| Rg3 | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and Rg3 | ↑ Nrf1, Pgc-1α, Tfam ↑ mitochondrial ATP content, MMP | ↑ mitochondrial biogenesis | [113] |
| 5,7-Dimethoxyflavone (DMF) | C57BL/6J mice | - young mice (11-week-old) - aged mice (19-month-old) - aged mice with DMF (for 8 weeks) | ↑ Nrf1, Pgc-1α, Tfam ↑ mtDNA content | ↑ mitochondrial biogenesis | [101] |
| Fucoxanthin | C2C12 myotube | - Non-treatment - Dex - Dex and fucoxanthin | ↑ Nrf1, Pgc-1α, Tfam ↓ acetylated Pgc-1α ↑ mitochondrial contents ↑ mitochondrial ATP content | ↑ mitochondrial biogenesis (Sirt1/Pgc-1α pathway) | [115] |
| Dihydromyricetin | L6 myotube | - Non-treatment - Dex - Dex and dihydromyricetin | ↑ mtDNA, Pgc-1α, Tfam ↓ mitochondrial morphological abnormality ↑ Mfn2, Tom20 ↓ change of MMP ↑ complex I and IV activity | ↑ mitochondrial biogenesis ↑ mitochondrial dynamics ↑ mitochondrial respiratory capacity | [118] |
| Rg3 | C2C12 myotube | - Non-treatment - Dex - Dex and Rg3 | ↑ mitochondrial OCR, ATP content, MMP ↓ mitochondrial morphological changes (swelling, increased volume) ↓ nuclear FoxO3, p-AMPK ↑ cytosolic FoxO3 | ↑ mitochondrial respiratory capacity (↓ AMPK/FoxO3 signaling pathway) | [119] |
| Probiotics | |||||
| Lactobacillus paracasei PS23 | SAMP8 mice | - non-aged mice (16-week-old) - aged mice (28-week-old) - aged mice with L. paracasei PS23 (for 12 weeks) | ↑ Nrf1, Pgc-1a, Tfam ↑ mtDNA copy number | ↑ mitochondrial biogenesis | [110] |
| Lactobacillus casei Shirota | SAMP8 mice | - non-aged mice (16-week-old) - aged mice (28-week-old) - aged mice with L. casei Shirota (for 12 weeks) | ↑ Sirt1, Tfam ↑ mtDNA copy number ↑ OCR | ↑ mitochondrial biogenesis ↑ mitochondrial respiratory capacity | [109] |
3.4. Reversal of SC Dysfunction by Natural Dietary Ingredients
| Natural Dietary Ingredients | Experimental Model | Experimental Design | Result 1 (by Natural Dietary Ingredient Treatment) | Related Mechanism (Potential Pathway) | Ref. |
|---|---|---|---|---|---|
| Botanical and marine extracts (bioactive compound) | |||||
| Lemon myrtle extract (casuarinin) | SD rats | - young rats (13-week-old) - young rats with Lemon myrtle extract or casuarinin (for 4 days) | ↑ BrdU incorporation into SCs | ↑ SC activation | [120] |
| Catechin-rich-green tea extract | C57BL/6JRj mice | - young mice (4-month-old) - aged mice (22-month-old) - aged mice with GTE (for 6 month) | ↑ Pax7-positive SCs | ↑ SC population | [114] |
| Phytochemicals | |||||
| Cocoa flavanols | C57BL/6JRj mice | - young mice (4-month-old) - aged mice (22-month-old) - aged mice with cocoa flavanols (for 6 month) | ↑ Pax7-positive SCs | ↑ SC population | [114] |
| Curcumin | C57BL/10ScSn mice | - young mice (6-month-old) - aged mice (18-month-old) - aged mice with curcumin (for 6 months) | ↑ myofiber regeneration after acute damage ↑ MyoD-positive SC | ↑ SC activation | [121] |
| Sinensetin | Isolated SCs from SD rats | - young rats (6-week-old) - aged rats (24-month-old) - aged rats with sinensetin (for 5 days) | ↑ myoblast differentiation of SC ↑ MyoD, myogenin | ↑ SC activation | [122] |
3.5. Improvement in Diagnosis Criteria and Biochemical Index for Sarcopenia by Natural Dietary Ingredients
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Lee, J.Y.; Gil, C.R.; Kim, M.K. Prevalence of Sarcopenia in Community-Dwelling Older Adults According to Simplified Algorithms for Sarcopenia Consensus Based on Asian Working Group for Sarcopenia. Clin. Interv. Aging 2020, 15, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing. Available online: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed on 21 July 2021).
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the Link between Lean Mass and Grip Strength Cut Points with Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1317–1323. [Google Scholar] [CrossRef]
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef]
- Freiberger, E.; Sieber, C.C.; Kob, R. Mobility in Older Community-Dwelling Persons: A Narrative Review. Front. Physiol. 2020, 11, 881. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, P.; Dou, Q.; Wang, C.; Zhang, W.; Yang, Y.; Wang, J.; Xie, X.; Zhou, J.; Zeng, Y. Falls among older adults with sarcopenia dwelling in nursing home or community: A meta-analysis. Clin. Nutr. 2020, 39, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Fang, F.; Li, F.; Ren, Y.; Hu, J.; Cao, J. Sarcopenia is associated with hypertension in older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 279. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Chai, Y.H.; Gong, H.J.; Zhuldyz, Z.; Stehouwer, C.D.A.; Zhou, J.B.; Simo, R. The Association Between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences From Observational Studies. Front. Endocrinol. 2021, 12, 782391. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wang, C.; Tao, W.; Dou, Q.; Yang, Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatr. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated with Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, X.; Lera, L.; Marquez, C.; Albala, C. Forecasting Healthy Life Expectancy Among Chilean Community-Dwelling Older Adults With and Without Sarcopenia. Front. Med. 2022, 9, 841810. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Sanchez, G.F.L.; Veronese, N.; Soysal, P.; Kostev, K.; Jacob, L.; Oh, H.; Tully, M.A.; Butler, L.; Parsa, A.D.; et al. Association between sarcopenia and quality of life among adults aged >/= 65 years from low- and middle-income countries. Aging Clin. Exp. Res. 2022, 34, 2779–2787. [Google Scholar] [CrossRef]
- Cao, L.; Morley, J.E. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J. Am. Med. Dir. Assoc. 2016, 17, 675–677. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, J.T.; Park, C.H.; Cha, Y. Diagnosis and Management of Sarcopenia after Hip Fracture Surgery: Current Concept Review. Hip Pelvis 2022, 34, 1–9. [Google Scholar] [CrossRef]
- Cesari, M.; Bernabei, R.; Vellas, B.; Fielding, R.A.; Rooks, D.; Azzolino, D.; Mariani, J.; Oliva, A.A.; Bhasin, S.; Rolland, Y. Challenges in the Development of Drugs for Sarcopenia and Frailty—Report from the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force. J. Frailty Aging 2022, 11, 135–142. [Google Scholar] [CrossRef]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med. J. 2012, 3, e0024. [Google Scholar] [CrossRef]
- Sun, F.; Norman, I.J.; While, A.E. Physical activity in older people: A systematic review. BMC Public Health 2013, 13, 449. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef] [Green Version]
- Cade, W.T.; Yarasheski, K.E. Metabolic and Molecular Aspects of Sarcopenia. In Principles of Molecular Medicine; Runge, M.S., Patterson, C., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 529–534. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Sattler, F.R. Growth hormone in the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Voges, D.; Zwickl, P.; Baumeister, W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999, 68, 1015–1068. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.P.; Hewitt, E.W. Cellular proteostasis: Degradation of misfolded proteins by lysosomes. Essays Biochem. 2016, 60, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The Role of Autophagy in Skeletal Muscle Diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [Green Version]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef]
- Marcell, T.J. Sarcopenia: Causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M911–M916. [Google Scholar] [CrossRef]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [Green Version]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Waters, D.L.; Qualls, C.R.; Dorin, R.I.; Veldhuis, J.D.; Baumgartner, R.N. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leung, K.S.; Chow, S.K.; Cheung, W.H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop. Transl. 2017, 10, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, T.; Liu, H.; Li, Z.; Peng, L.; Wang, C.; Wang, T. Inflammaging: The ground for sarcopenia? Exp. Gerontol. 2022, 168, 111931. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Li, G.X.; Jiang, L.J.; Yu, S.L.; Xu, L.Y.; Liu, R.J.; Guo, Z.J.; Xie, H.Y.; et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J. Cachexia Sarcopenia Muscle 2019, 10, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Sciorati, C.; Gamberale, R.; Monno, A.; Citterio, L.; Lanzani, C.; De Lorenzo, R.; Ramirez, G.A.; Esposito, A.; Manunta, P.; Manfredi, A.A.; et al. Pharmacological blockade of TNFalpha prevents sarcopenia and prolongs survival in aging mice. Aging 2020, 12, 23497–23508. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [Green Version]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosicki, G.J.; Barrett, B.B.; Englund, D.A.; Liu, C.; Travison, T.G.; Cederholm, T.; Koochek, A.; von Berens, A.; Gustafsson, T.; Benard, T.; et al. Circulating Interleukin-6 Is Associated with Skeletal Muscle Strength, Quality, and Functional Adaptation with Exercise Training in Mobility-Limited Older Adults. J. Frailty Aging 2020, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, N.; Wang, J.; Ladner, K.J.; Wang, H.; Dahlman, J.M.; Carathers, M.; Acharyya, S.; Rudnicki, M.A.; Hollenbach, A.D.; Guttridge, D.C. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J. Cell Biol. 2008, 180, 787–802. [Google Scholar] [CrossRef] [Green Version]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [CrossRef]
- Viner, R.I.; Ferrington, D.A.; Williams, T.D.; Bigelow, D.J.; Schoneich, C. Protein modification during biological aging: Selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem. J. 1999, 340 Pt 3, 657–669. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Qaisar, R.; Pharaoh, G.; Bhaskaran, S.; Xu, H.; Ranjit, R.; Bian, J.; Ahn, B.; Georgescu, C.; Wren, J.D.; Van Remmen, H. Restoration of Sarcoplasmic Reticulum Ca(2+) ATPase (SERCA) Activity Prevents Age-Related Muscle Atrophy and Weakness in Mice. Int. J. Mol. Sci. 2020, 22, 37. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, C.; Thompson, L. Sarcopenia of ageing: Functional, structural and biochemical alterations. Braz. J. Phys. 2007, 11, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Arnold, P.; Njemini, R.; Vantieghem, S.; Duchateau, J.; Mets, T.; Beyer, I.; Bautmans, I. Peripheral muscle fatigue in hospitalised geriatric patients is associated with circulating markers of inflammation. Exp. Gerontol. 2017, 95, 128–135. [Google Scholar] [CrossRef]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef] [Green Version]
- Zahn, J.M.; Sonu, R.; Vogel, H.; Crane, E.; Mazan-Mamczarz, K.; Rabkin, R.; Davis, R.W.; Becker, K.G.; Owen, A.B.; Kim, S.K. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006, 2, e115. [Google Scholar] [CrossRef]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Fan, Y.B.; Tao, X.H.; Pan, W.L.; Wu, Y.X.; Wang, X.H.; He, Y.Q.; Xiao, W.F.; Li, Y.S. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2021, 12, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Chang, Y.C.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology 2020, 21, 367–380. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.; Ostojic, O.; Singh, K.; Joseph, A.M.; Hood, D.A. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 2013, 48, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Lorenzi, M.; Tanganelli, F.; Picca, A.; Bossola, M.; Menghi, A.; Bernabei, R.; Landi, F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2016, 80, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Parise, G. Role of muscle stem cells in sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 186–190. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, D.; Yang, Y.; Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Xiao, W.; Li, Y. The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem Cell Res. Ther. 2022, 13, 28. [Google Scholar] [CrossRef]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Q.; Liu, H. Contribution of muscle satellite cells to sarcopenia. Front. Physiol. 2022, 13, 892749. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef] [Green Version]
- Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014, 20, 265–271. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Victor, P.; Munoz-Canoves, P. Regenerative decline of stem cells in sarcopenia. Mol. Asp. Med. 2016, 50, 109–117. [Google Scholar] [CrossRef]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortes-Lopez, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. eLife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwee, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; The Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Lee, S.; Kim, K. The effect of combining nutrient intake and physical activity levels on central obesity, sarcopenia, and sarcopenic obesity: A population-based cross-sectional study in South Korea. BMC Geriatr. 2023, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J.; et al. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2011, 123, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar] [CrossRef]
- Hong, K.B.; Lee, H.S.; Kim, D.H.; Moon, J.M.; Park, Y. Tannase-Converted Green Tea Extract with High (-)-Epicatechin Inhibits Skeletal Muscle Mass in Aged Mice. Evid Based Complement. Altern. Med. 2020, 2020, 4319398. [Google Scholar] [CrossRef] [Green Version]
- Salvadori, L.; Mandrone, M.; Manenti, T.; Ercolani, C.; Cornioli, L.; Lianza, M.; Tomasi, P.; Chiappalupi, S.; Di Filippo, E.S.; Fulle, S.; et al. Identification of Withania somnifera-Silybum marianum-Trigonella foenum-graecum Formulation as a Nutritional Supplement to Contrast Muscle Atrophy and Sarcopenia. Nutrients 2020, 13, 49. [Google Scholar] [CrossRef]
- Lee, J.; Kim, C.; Lee, H.; Hwang, J.K. Inhibitory Effects of Standardized Leonurus japonicus Extract and Its Bioactive Leonurine on TNF-alpha-Induced Muscle Atrophy in L6 Myotubes. J. Microbiol. Biotechnol. 2020, 30, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Kim, C.; Woo, Y.K.; Hwang, J.K. Inhibitory Effects of Chrysanthemum (Chrysanthemum morifolium Ramat.) Extract and Its Active Compound Isochlorogenic Acid A on Sarcopenia. Prev. Nutr. Food Sci. 2021, 26, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chen, Y.T.; Liu, H.W.; Chan, Y.C.; Liu, M.Y.; Hu, S.H.; Tseng, W.T.; Wu, H.L.; Wang, M.F.; Chang, S.J. Oligonol Alleviates Sarcopenia by Regulation of Signaling Pathways Involved in Protein Turnover and Mitochondrial Quality. Mol. Nutr. Food Res. 2019, 63, e1801102. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Kim, M.; Choi, S.Y.; Jang, Y.; Ha, T.Y. Isobavachalcone attenuates myotube atrophy induced by TNF-alpha through muscle atrophy F-box signaling and the nuclear factor erythroid 2-related factor 2 cascade. Phytother Res. 2019, 33, 403–411. [Google Scholar] [CrossRef]
- Han, Y.; Lee, H.; Li, H.; Ryu, J.H. Corylifol A from Psoralea corylifolia L. Enhances Myogenesis and Alleviates Muscle Atrophy. Int. J. Mol. Sci. 2020, 21, 1571. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, M.; Kitakaze, T.; Kobayashi, Y.; Mukai, K.; Harada, N.; Yamaji, R. beta-Cryptoxanthin Improves p62 Accumulation and Muscle Atrophy in the Soleus Muscle of Senescence-Accelerated Mouse-Prone 1 Mice. Nutrients 2020, 12, 2180. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Park, S.D.; Shim, J.J.; Lee, J.L. Lactobacillus plantarum HY7715 Ameliorates Sarcopenia by Improving Skeletal Muscle Mass and Function in Aged Balb/c Mice. Int. J. Mol. Sci. 2021, 22, 10023. [Google Scholar] [CrossRef]
- Winbanks, C.E.; Weeks, K.L.; Thomson, R.E.; Sepulveda, P.V.; Beyer, C.; Qian, H.; Chen, J.L.; Allen, J.M.; Lancaster, G.I.; Febbraio, M.A.; et al. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012, 197, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Hwang, J.K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef]
- Morel, S.; Hugon, G.; Vitou, M.; Vedere, M.; Fons, F.; Rapior, S.; Saint, N.; Carnac, G. A Bioassay-Guided Fractionation of Rosemary Leaf Extract Identifies Carnosol as a Major Hypertrophy Inducer in Human Skeletal Muscle Cells. Nutrients 2021, 13, 4190. [Google Scholar] [CrossRef]
- Oh, H.J.; Jin, H.; Lee, B.Y. The non-saponin fraction of Korean Red Ginseng ameliorates sarcopenia by regulating immune homeostasis in 22-26-month-old C57BL/6J mice. J. Ginseng Res. 2022, 46, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hedstrom, D.; Priego, T.; Amor, S.; de la Fuente-Fernandez, M.; Martin, A.I.; Lopez-Calderon, A.; Inarejos-Garcia, A.M.; Garcia-Villalon, A.L.; Granado, M. Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle. Antioxidants 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Yang, I.-H.; Lin, Y.-W.; Lin, J.-N.; Wu, C.-C.; Chiang, C.-Y.; Lai, K.-H.; Lin, F.-H. Curcumin-Loaded Hydrophobic Surface-Modified Hydroxyapatite as an Antioxidant for Sarcopenia Prevention. Antioxidants 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis protein protects against TNFalphainduced myotube atrophy in C2C12 myotubes via the NFkappaB signaling pathway. Mol. Med. Rep. 2021, 24, 486. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ahn, G.; Kim, H.S.; Je, J.G.; Kim, K.N.; Jeon, Y.J. Diphlorethohydroxycarmalol (DPHC) Isolated from the Brown Alga Ishige okamurae Acts on Inflammatory Myopathy as an Inhibitory Agent of TNF-α. Mar. Drugs 2020, 18, 529. [Google Scholar] [CrossRef]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef]
- Chen, L.H.; Huang, S.Y.; Huang, K.C.; Hsu, C.C.; Yang, K.C.; Li, L.A.; Chan, C.H.; Huang, H.Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging 2019, 11, 756–770. [Google Scholar] [CrossRef]
- Lee, S.J.; Im, M.; Park, S.K.; Kim, J.Y.; So, E.Y.; Liang, O.D.; Kang, J.S.; Bae, G.U. BST204, a Rg3 and Rh2 Enriched Ginseng Extract, Upregulates Myotube Formation and Mitochondrial Function in TNF-α-Induced Atrophic Myotubes. Am. J. Chin. Med. 2020, 48, 631–650. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.; Im, M.; Park, S.K.; Han, H.J.; An, S.; Kang, J.S.; Lee, S.J.; Bae, G.U. BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function. Int. J. Environ. Res. Public Health 2021, 18, 2367. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, J.H.; Lee, H.; Lee, H.; Park, J.; Kang, J.S.; Bae, G.U. Ginsenoside Rg3 upregulates myotube formation and mitochondrial function, thereby protecting myotube atrophy induced by tumor necrosis factor-alpha. J. Ethnopharmacol. 2019, 242, 112054. [Google Scholar] [CrossRef] [PubMed]
- Gras, S.; Blasco, A.; Mòdol-Caballero, G.; Tarabal, O.; Casanovas, A.; Piedrafita, L.; Barranco, A.; Das, T.; Rueda, R.; Pereira, S.L.; et al. Beneficial effects of dietary supplementation with green tea catechins and cocoa flavanols on aging-related regressive changes in the mouse neuromuscular system. Aging 2021, 13, 18051–18093. [Google Scholar] [CrossRef] [PubMed]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharm. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.I.; Kim, M.J.; Hahm, J.H.; Seo, H.D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Castor Oil Plant (Ricinus communis L.) Leaves Improve Dexamethasone-Induced Muscle Atrophy via Nrf2 Activation. Front. Pharm. 2022, 13, 891762. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.I.; Nirmala, F.S.; Jeong, H.Y.; Seo, H.D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Chrysanthemum zawadskil Herbich attenuates dexamethasone-induced muscle atrophy through the regulation of proteostasis and mitochondrial function. Biomed. Pharm. 2021, 136, 111226. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1α Pathway. Cell Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, R.; Liu, J.; Xu, X.; Sun, G.; Zhao, D.; Sun, L. 20(s)-ginseonside-Rg3 modulation of AMPK/FoxO3 signaling to attenuate mitochondrial dysfunction in a dexamethasone-injured C2C12 myotube-based model of skeletal atrophy in vitro. Mol. Med. Rep. 2021, 23, 620. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honda, S.; Ogura, M.; Kato, M.; Tanigawa, R.; Fujino, H.; Kawamoto, S. Lemon Myrtle (Backhousia citriodora) Extract and Its Active Compound, Casuarinin, Activate Skeletal Muscle Satellite Cells In Vitro and In Vivo. Nutrients 2022, 14, 1078. [Google Scholar] [CrossRef]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondi, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.M.; Ha, S.E.; Vetrivel, P.; Saralamma, V.V.G.; Kim, E.H.; Kim, G.S. Sinensetin regulates age-related sarcopenia in cultured primary thigh and calf muscle cells. BMC Complement. Altern. Med. 2019, 19, 287. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Lee, S.H.; Park, Y.; Lee, H.S.; Hong, J.S.; Lim, C.Y.; Kim, D.H.; Park, S.S.; Suh, H.J.; Hong, K.B. (-)-Epicatechin-Enriched Extract from Camellia sinensis Improves Regulation of Muscle Mass and Function: Results from a Randomized Controlled Trial. Antioxidants 2021, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Rheu, K.M.; Lee, B.J.; Son, W.H.; Kim, D.S.; Park, H.T.; Ha, M.S.; Gong, B.H.; Jeon, B.H. Effect of Fermented Sarco Oyster (Crassostrea gigas) Extract on Muscle Strength Enhancement in Postmenopausal Females: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16450. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.F.; Jackman, S.R.; Sabou, V.R.; Campbell, M.I.; Tang, J.C.Y.; Dutton, J.; Bowtell, J.L. Shatavari Supplementation in Postmenopausal Women Improves Handgrip Strength and Increases Vastus lateralis Myosin Regulatory Light Chain Phosphorylation but Does Not Alter Markers of Bone Turnover. Nutrients 2021, 13, 4282. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Ryu, B.; Oh, S.; Chung, D.M.; Seo, M.; Park, S.J.; Byun, K.; Jeon, Y.J. Reversibility of sarcopenia by Ishige okamurae and its active derivative diphloroethohydroxycarmalol in female aging mice. Biomed. Pharm. 2022, 152, 113210. [Google Scholar] [CrossRef]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef]
- Lee, M.C.; Tu, Y.T.; Lee, C.C.; Tsai, S.C.; Hsu, H.Y.; Tsai, T.Y.; Liu, T.H.; Young, S.L.; Lin, J.S.; Huang, C.C. Lactobacillus plantarum TWK10 Improves Muscle Mass and Functional Performance in Frail Older Adults: A Randomized, Double-Blind Clinical Trial. Microorganisms 2021, 9, 1466. [Google Scholar] [CrossRef]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- El Abed, N.; Ozogul, F. The risks of marine micro/nano-plastics on seafood safety and human health. Adv. Food Nutr. Res. 2023, 103, 229–271. [Google Scholar] [CrossRef]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367. [Google Scholar] [CrossRef] [Green Version]



| Natural Dietary Ingredients | Experimental Model | Experimental Designs | Results 1 (by Natural Dietary Ingredient Treatment) | Related Mechanisms (Potential Pathway) | Ref. |
|---|---|---|---|---|---|
| Botanical and marine extracts (bioactive compound) | |||||
| Tannase-converted green tea extract | ICR mice | - aged mice (24-month-old) - aged mice with tannase-converted green tea extract | ↑ MyoD, myogenin ↓ p-FoxO3a, myostatin, MuRF1, atrogin-1 ↑ lean mass | ↑ myogenesis ↓ protein degradation (UPS; FoxO3 pathway) | [91] |
| WST | C2C12 myotube | - PBS and WST - TNF-α/IFNγ and WST - Dex and WST | ↑ myogenin, p-p38 ↑ myotube diameter ↑ the number of nuclei inside myotube, total nuclei ↑ MHC type II, p-Akt | ↑ myogenesis (p38 MAPK/myogenin pathway) ↑ protein synthesis (PI3K/Akt pathway) | [92] |
| Human (Vastus lateralis muscle biopsies) | - young subjects - sarcopenic subjects | ↑ myotube diameter ↑ the number of nuclei inside myotube ↑ MHC expression | ↑ myogenesis | ||
| Leonurus japonicus extract (leonurine) | L6 myotube | - Non-treatment - TNF-α and L. japonicus extract or leonurine | ↑ p-PI3K, p-Akt, p-mTOR, p-p70S6K, p-4E-BP1 ↑ p-FoxO3 ↓ atrogin-1, MuRF1 ↑ myotube diameter | ↑ protein synthesis (PI3K/Akt pathway) ↓ protein degradation (UPS; FoxO3 pathway) | [93] |
| Chrysanthemum morifolium Ramat. extract (isochlorogenic acid A) | L6 myotube | - Non-treatment - TNF-α - TNF-α and C. morifolium Ramat. extract or isochlorogenic acid A | ↑ p-PI3K, p-Akt, p-mTOR, p-p70S6K, p-4E-BP1 ↓ p-FoxO3 ↓ atrogin-1, MuRF1 | ↑ protein synthesis (PI3K/Akt pathway) ↓ protein degradation (UPS; FoxO3 pathway) | [94] |
| Oligonol | SAMP8 mice | - SAMR1 control mice (32-week-old) - SAMP8 mice (32-week-old) - SAMP8 mice with oligonol (for 8 weeks) | ↑ p-Akt, p-mTOR, p-p70S6K ↓ nuclear localization of FoxO3a and NF-κB ↓ atrogin-1, MuRF1 ↓ Atg13, LC3-II, p62 ↑ CSA, grip strength, muscle mass | ↑ protein synthesis (PI3K/Akt pathway) ↓ protein degradation (UPS; FoxO3 pathway, autophagy) | [95] |
| Phytochemicals | |||||
| Isobavachalcone | C2C12 myotube | - Non-treatment - TNF-α - TNF-α and isobavachalcone | ↑ myogenin, MyoD, MHC, myotube diameter ↓ atrogin-1, MuRF1, p-FoxO1 | ↑ myogenesis ↓ protein degradation (UPS) | [96] |
| Corylifol A | C2C12 myotube | - Non-treatment - Dex - Dex and corylifol A | ↑ p-Akt, MHC ↓ atrogin-1, MuRF1, myostatin ↑ number of multinucleated myotube | ↑ protein synthesis ↓ protein degradation (UPS) | [97] |
| β-Cryptoxanthin | SAMP1 mice | - SAMR1 control mice (20-week-old) - SAMP1 mice (20-week-old) - SAMP1 mice with β-Cryptoxanthin (for 15 weeks) | ↓ beclin1, p62, LC3-I, LC3-II ↑ mass, CSA, MHC type I (soleus) | ↓ protein degradation (autophagy) ↑ muscle hypertrophy | [98] |
| Probiotics | |||||
| Lactobacillus plantarum HY7715 | Balb/c mice | - young mice (7-week-old) - aged mice (81-week-old) - aged mice with HY7715 (for 5 weeks) | ↓ atrogin-1, MuRF1 ↑ mass (soleus, gastrocnemius) ↑ CSA, MyoD, MHC type I | ↓ protein degradation (UPS) ↑ muscle hypertrophy | [99] |
| Natural Dietary Ingredients | Experimental Model | Experimental Designs | Treatment | Criteria for Sarcopenia 1 (Mass/Strength/Physical Performance) | Biochemical Index 2 | Ref. | |
|---|---|---|---|---|---|---|---|
| Botanical and marine extracts (bioactive compound) | |||||||
| Tannase-treated green tea extract | Older adults (>60 yr, 10 males and 57 females) | - placebo-control (n = 34) - Tannase-treated green tea extract (n = 33) | 600 mg/d for 12 weeks | Mass | = body muscle mass | = follistatin, CRP, IL-6, IL-8, IGF-1, cortisol ↓ myostatin | [123] |
| Strength | ↑ isokinetic flexor strength (right leg), handgrip strength | ||||||
| Physical performance | ND | ||||||
| Fermented sarco oysters extract | Postmenopausal women with low muscle mass (> 65 yr) | - placebo-control (n = 23) - Fermented sarco oysters extract (n = 23) | 1000 mg/d for 12 weeks | Mass | = appendicular skeletal mass/height2 | = CRP ↑ IGF-1 | [124] |
| Strength | ↑ quadriceps muscle strength, handgrip strength | ||||||
| Physical performance | ND | ||||||
| Shatavari | Postmenopausal women with low muscle mass (> 60 yr) | - placebo-control (n = 10) - Shatavari (n = 10) | 1000 mg/d for 6 weeks | Mass | ND | ND | [125] |
| Strength | ↑ handgrip strength = knee extensor strength | ||||||
| Physical performance | ND | ||||||
| Ishige okamurae (diphlorethohydroxycarmalol) | C57BL/6J mice | - young mice (4-month-old) - aged mice (14-month-old) - aged mice with I. okamurae | 50–200 mg/kg/d for 7 weeks | Mass | ↑ lean mass | ↓ IL-1β, IL-6, TNF-α | [126] |
| Strength | ↑ grip strength | ||||||
| Physical performance | ↓ the required time for ladder climbing | ||||||
| Phytochemicals | |||||||
| Cur-SHAP | SD rats | - control rats with saline (12-month-old) - sarcopenic rats with LPS - sarcopenic rats with LPS + Cur-SHAP | 150 mg/kg/d 4 times for 8 weeks | Mass | ↓ fat/lean mass ratio (%) | ↓ creatine kinase, lactate dehydrogenase, ALT = total protein, calcium | [105] |
| Strength | ↑ grip strength | ||||||
| Physical performance | ↑ muscle endurance by treadmill test | ||||||
| Cureit | Older adults (>65 yr, 13 males and 17 females) | - placebo-control (n = 15) - Cureit (n = 15) | 500 mg/d for 12 weeks | Mass | ND | ND | [127] |
| Strength | ↑ handgrip strength | ||||||
| Physical performance | ND | ||||||
| Probiotics | |||||||
| Lactobacillus plantarum TWK10 | Older adults with frailty (55–85 yr, 25 males and 17 females) | - placebo-control (n = 17) - low-dose TWK10 (n = 12) - high-dose TWK10 (n = 13) | 2 × 1010 or 13; 6 × 1010 CFU/day for 18 weeks | Mass | ↑ muscle mass | ND | [128] |
| Strength | ↑ handgrip strength | ||||||
| Physical performance | ↑ gait and balance ability ↑ the walking ability, lower-limb muscle strength, and endurance | ||||||
| Lactobacillus plantarum HY7715 | Balb/c mice | - young mice (7-week-old) - aged mice (81-week-old) - aged mice with HY7715 | 1 × 108 CFU/kg /day for 5 weeks | Mass | ↑ muscle weight (soleus, gastrocnemius) | ↓ lactate, BUN, creatinine = AST, ALT | [99] |
| Strength | ↑ grip strength (fore and all limb) | ||||||
| Physical performance | ↑ running endurance (treadmill distance) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, J.-Y.; Kim, C.Y. A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia. Nutrients 2023, 15, 2625. https://doi.org/10.3390/nu15112625
Kim J, Lee J-Y, Kim CY. A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia. Nutrients. 2023; 15(11):2625. https://doi.org/10.3390/nu15112625
Chicago/Turabian StyleKim, Juhae, Joo-Yeon Lee, and Choon Young Kim. 2023. "A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia" Nutrients 15, no. 11: 2625. https://doi.org/10.3390/nu15112625