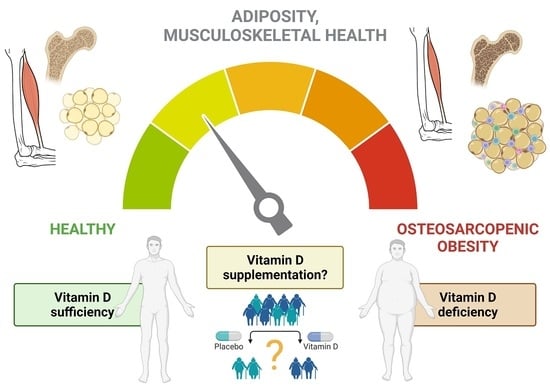
Vitamin D in Osteosarcopenic Obesity
Abstract
:1. Introduction
2. Osteosarcopenic Obesity: Prevalence, Risk Factors and Impact on Health Outcomes
3. Pathogenic Mechanisms Underlying Osteosarcopenic Obesity
3.1. Role of Endocrine Dysregulation
3.2. Low-Grade Chronic Inflammation
4. Hypovitaminosis D and Osteosarcopenic Obesity
4.1. Vitamin D: Brief Overview
4.2. Vitamin D, Insulin Resistance and Musculoskeletal Health
4.3. Vitamin D in Osteosarcopenic Obesity: Is There a Therapeutic Role?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, P.; Body, J.J.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Kaufman, J.; Reginster, J.Y.; Rozenberg, S. Loading and skeletal development and maintenance. J. Osteoporos. 2010, 2011, 786752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Turcotte, A.F.; O’Connor, S.; Morin, S.N.; Gibbs, J.C.; Willie, B.M.; Jean, S.; Gagnon, C. Association between obesity and risk of fracture, bone mineral density and bone quality in adults: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252487. [Google Scholar] [CrossRef]
- Gandham, A.; Mesinovic, J.; Jansons, P.; Zengin, A.; Bonham, M.P.; Ebeling, P.R.; Scott, D. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13187. [Google Scholar] [CrossRef]
- Scott, D.; Chandrasekara, S.D.; Laslett, L.L.; Cicuttini, F.; Ebeling, P.R.; Jones, G. Associations of Sarcopenic Obesity and Dynapenic Obesity with Bone Mineral Density and Incident Fractures Over 5-10 Years in Community-Dwelling Older Adults. Calcif. Tissue Int. 2016, 99, 30–42. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, Y.; Yu, X.; Liu, J.; Long, Q.; Zeng, Y.; Lu, S. An analysis and systematic review of sarcopenia increasing osteopenia risk. PLoS ONE 2021, 16, e0250437. [Google Scholar] [CrossRef]
- Sowers, M.F.; Kshirsagar, A.; Crutchfield, M.M.; Updike, S. Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am. J. Epidemiol. 1992, 136, 257–265. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachexia Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Cruz-Jentoft, A.J.; Fielding, R.A.; Kanis, J.A.; Reginster, J.Y.; Bruyere, O.; Cesari, M.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; et al. Is There Enough Evidence for Osteosarcopenic Obesity as a Distinct Entity? A Critical Literature Review. Calcif. Tissue Int. 2019, 105, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, M.M.; Charlton, K.; Thakur, S.; Ribeiro, H.; Lanham-New, S.A. Future perspectives in addressing the global issue of vitamin D deficiency. Proc. Nutr. Soc. 2020, 79, 246–251. [Google Scholar] [CrossRef]
- Zhang, S.; Miller, D.D.; Li, W. Non-Musculoskeletal Benefits of Vitamin D beyond the Musculoskeletal System. Int. J. Mol. Sci. 2021, 22, 2128. [Google Scholar] [CrossRef] [PubMed]
- Kolbasi, E.N.; Demirdag, F. Prevalence of osteosarcopenic obesity in community-dwelling older adults: A cross-sectional retrospective study. Arch. Osteoporos. 2020, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Inglis, J.E.; Kelly, O.J.; McGee, D.L. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos. Int. 2015, 26, 2587–2595. [Google Scholar] [CrossRef]
- Szlejf, C.; Parra-Rodriguez, L.; Rosas-Carrasco, O. Osteosarcopenic Obesity: Prevalence and Relation with Frailty and Physical Performance in Middle-Aged and Older Women. J. Am. Med. Dir. Assoc. 2017, 18, 733.e1–733.e5. [Google Scholar] [CrossRef]
- Pang, B.W.J.; Wee, S.L.; Chen, K.K.; Lau, L.K.; Jabbar, K.A.; Seah, W.T.; Ng, D.H.M.; Tan, Q.L.L.; Jagadish, M.U.; Ng, T.P. Coexistence of osteoporosis, sarcopenia and obesity in community-dwelling adults—The Yishun Study. Osteoporos. Sarcopenia 2021, 7, 17–23. [Google Scholar] [CrossRef]
- Stefanaki, C.; Peppa, M.; Boschiero, D.; Chrousos, G.P. Healthy overweight/obese youth: Early osteosarcopenic obesity features. Eur. J. Clin. Investig. 2016, 46, 767–778. [Google Scholar] [CrossRef]
- Fahimfar, N.; Zahedi Tajrishi, F.; Gharibzadeh, S.; Shafiee, G.; Tanha, K.; Heshmat, R.; Nabipour, I.; Raeisi, A.; Jalili, A.; Larijani, B.; et al. Prevalence of Osteosarcopenia and Its Association with Cardiovascular Risk Factors in Iranian Older People: Bushehr Elderly Health (BEH) Program. Calcif. Tissue Int. 2020, 106, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Chang, Y.M.; Kung, C.Y.; Sung, C.K.; Foo, W.S.; Wu, M.H.; Chiou, S.J. A study of correlations between metabolic syndrome factors and osteosarcopenic adiposity. BMC Endocr. Disord. 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Bae, Y.J. Dietary calcium, phosphorus, and osteosarcopenic adiposity in Korean adults aged 50 years and older. Arch. Osteoporos. 2021, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Bae, Y.J. Protein intake and osteosarcopenic adiposity in Korean adults aged 50 years and older. Osteoporos. Int. 2020, 31, 2363–2372. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, J.H.; Chon, D.; Lee, K.E. Diet quality and osteosarcopenic obesity in community-dwelling adults 50 years and older. Maturitas 2017, 104, 73–79. [Google Scholar] [CrossRef]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating Irisin Levels as a Marker of Osteosarcopenic-Obesity in Cushing’s Disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1565–1574. [Google Scholar] [CrossRef]
- Piccoli, A.; Cannata, F.; Strollo, R.; Pedone, C.; Leanza, G.; Russo, F.; Greto, V.; Isgro, C.; Quattrocchi, C.C.; Massaroni, C.; et al. Sclerostin Regulation, Microarchitecture, and Advanced Glycation End-Products in the Bone of Elderly Women with Type 2 Diabetes. J. Bone Miner. Res. 2020, 35, 2415–2422. [Google Scholar] [CrossRef]
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421–445. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Conte, C.; Secchi, A. Post-transplantation diabetes in kidney transplant recipients: An update on management and prevention. Acta Diabetol. 2018, 55, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; Cinel, E.; Falbo, E.; Ferrante, M.; Cilla, M.; Martinenghi, S.; Vitali, G.; Bosi, E.; Giustina, A.; et al. Weight trajectories and abdominal adiposity in COVID-19 survivors with overweight/obesity. Int. J. Obes. 2021, 45, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr. J. 2014, 61, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [Green Version]
- Lee, K. Association of osteosarcopenic obesity and its components: Osteoporosis, sarcopenia and obesity with insulin resistance. J. Bone Miner. Metab. 2020, 38, 695–701. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.; Won, Y.J.; Kim, S.H. Clinical Manifestations and Factors Associated with Osteosarcopenic Obesity Syndrome: A Cross-Sectional Study in Koreans with Obesity. Calcif. Tissue Int. 2019, 105, 77–88. [Google Scholar] [CrossRef]
- Mo, D.; Hsieh, P.; Yu, H.; Zhou, L.; Gong, J.; Xu, L.; Liu, P.; Chen, G.; Chen, Z.; Deng, Q. Osteosarcopenic obesity and its relationship with dyslipidemia in women from different ethnic groups of China. Arch. Osteoporos. 2018, 13, 65. [Google Scholar] [CrossRef]
- Chen, X.; Kong, C.; Yu, H.; Gong, J.; Lan, L.; Zhou, L.; Gong, J.; Liu, P.; Xu, L.; Deng, Q. Association between osteosarcopenic obesity and hypertension among four minority populations in China: A cross-sectional study. BMJ Open 2019, 9, e026818. [Google Scholar] [CrossRef] [Green Version]
- Perez-Tasigchana, R.F.; Leon-Munoz, L.M.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Laclaustra, M.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 2017, 46, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zhang, W.; Han, P.; Kohzuki, M.; Guo, Q. Osteosarcopenic Obesity Associated with Poor Physical Performance in the Elderly Chinese Community. Clin. Interv. Aging 2020, 15, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, A.; Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018, 6, 743–752. [Google Scholar] [CrossRef]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Cesari, M.; Mari, D. Aging of the endocrine system and its potential impact on sarcopenia. Eur. J. Intern. Med. 2016, 35, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Attaix, D.; Mosoni, L.; Dardevet, D.; Combaret, L.; Mirand, P.P.; Grizard, J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int. J. Biochem. Cell Biol. 2005, 37, 1962–1973. [Google Scholar] [CrossRef]
- Ferrau, F.; Korbonits, M. Metabolic Syndrome in Cushing’s Syndrome Patients. Front. Horm. Res. 2018, 49, 85–103. [Google Scholar] [CrossRef]
- Frara, S.; Allora, A.; di Filippo, L.; Formenti, A.M.; Loli, P.; Polizzi, E.; Tradati, D.; Ulivieri, F.M.; Giustina, A. Osteopathy in mild adrenal Cushing’s syndrome and Cushing disease. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101515. [Google Scholar] [CrossRef]
- Carvalhaes-Neto, N.; Huayllas, M.K.; Ramos, L.R.; Cendoroglo, M.S.; Kater, C.E. Cortisol, DHEAS and aging: Resistance to cortisol suppression in frail institutionalized elderly. J. Endocrinol. Investig. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Kumari, M.; Badrick, E.; Sacker, A.; Kirschbaum, C.; Marmot, M.; Chandola, T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology 2010, 35, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.Y.; Rao, T.S.; Narayanaswamy, R.K. Study of hypothalamo pituitary adrenal axis in frail elderly subjects. J. Assoc. Phys. India 2012, 60, 31–34. [Google Scholar]
- Prokopidis, K.; Dionyssiotis, Y. Effects of sleep deprivation on sarcopenia and obesity: A narrative review of randomized controlled and crossover trials. J. Frailty Sarcopenia Falls 2021, 6, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Qualls, C.R.; Dorin, R.I.; Veldhuis, J.D.; Baumgartner, R.N. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/ IGF- 1 Status in Obese Subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef] [Green Version]
- Stanworth, R.D.; Jones, T.H. Testosterone for the aging male; current evidence and recommended practice. Clin. Interv. Aging 2008, 3, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am. J. Physiol. 1995, 269, E820–E826. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Allan, C.A.; Strauss, B.J.; Burger, H.G.; Forbes, E.A.; McLachlan, R.I. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J. Clin. Endocrinol. Metab. 2008, 93, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Formenti, A.M.; Dalla Volta, A.; di Filippo, L.; Berruti, A.; Giustina, A. Effects of Medical Treatment of Prostate Cancer on Bone Health. Trends Endocrinol. Metab. 2021, 32, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Dhindsa, S.; Batra, M.; Green, K.; Abuaysheh, S.; Kuhadiya, N.D.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Effect of Testosterone on FGF2, MRF4, and Myostatin in Hypogonadotropic Hypogonadism: Relevance to Muscle Growth. J. Clin. Endocrinol. Metab. 2019, 104, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, L.J.; Gupta, N.; Bhasin, M.; Singh, A.B.; Ross, R.; Phillips, J.; Bhasin, S. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J. Clin. Endocrinol. Metab. 2004, 89, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Snyder, P.J.; Kopperdahl, D.L.; Stephens-Shields, A.J.; Ellenberg, S.S.; Cauley, J.A.; Ensrud, K.E.; Lewis, C.E.; Barrett-Connor, E.; Schwartz, A.V.; Lee, D.C.; et al. Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. JAMA Intern. Med. 2017, 177, 471–479. [Google Scholar] [CrossRef]
- Corona, G.; Maseroli, E.; Rastrelli, G.; Isidori, A.M.; Sforza, A.; Mannucci, E.; Maggi, M. Cardiovascular risk associated with testosterone-boosting medications: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2014, 13, 1327–1351. [Google Scholar] [CrossRef]
- Arun, C.P. Fight or flight, forbearance and fortitude: The spectrum of actions of the catecholamines and their cousins. Ann. N. Y. Acad. Sci. 2004, 1018, 137–140. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Gilman, J.C.; Cvijetic, S.; Boschiero, D. Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation. Nutrients 2020, 12, 989. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, G. HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arch. Ind. Hyg. Toxicol. 2014, 65, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef]
- Endres, S. Messengers and mediators: Interactions among lipids, eicosanoids, and cytokines. Am. J. Clin. Nutr. 1993, 57, 798S–800S. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef]
- Canavan, B.; Salem, R.O.; Schurgin, S.; Koutkia, P.; Lipinska, I.; Laposata, M.; Grinspoon, S. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J. Clin. Endocrinol. Metab. 2005, 90, 5779–5785. [Google Scholar] [CrossRef] [Green Version]
- Fruhbeck, G.; Catalan, V.; Rodriguez, A.; Gomez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Sciorati, C.; Capobianco, A.; Lore, N.I.; Giustina, A.; Manfredi, A.A.; Rovere-Querini, P.; Conte, C. Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab. 2021, 47, 101268. [Google Scholar] [CrossRef]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.; Chapurlat, R.; Ecochard, R.; Szulc, P. Low Muscle Strength and Mass Is Associated With the Accelerated Decline of Bone Microarchitecture at the Distal Radius in Older Men: The Prospective STRAMBO Study. J. Bone Miner. Res. 2018, 33, 1630–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, P.P.; Whittier, D.E.; Foesser, D.; Boyd, S.K.; Chapurlat, R.; Szulc, P. Bone Microarchitecture Decline and Risk of Fall and Fracture in Men With Poor Physical Performance-The STRAMBO Study. J. Clin. Endocrinol. Metab. 2021, 106, e5180–e5194. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Lu, R.; Zhang, Y.; Tao, H.; Chen, S. Relationship between oseteoporosis with fatty infiltration of paraspinal muscles based on QCT examination. J. Bone Miner. Metab. 2022. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [Green Version]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in Vitamin D: A Statement From the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Murayama, A.; Takeyama, K.; Kitanaka, S.; Kodera, Y.; Kawaguchi, Y.; Hosoya, T.; Kato, S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology 1999, 140, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Blau, J.E.; Collins, M.T. The PTH-Vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Maddaloni, E.; Cavallari, I.; Napoli, N.; Conte, C. Vitamin D and Diabetes Mellitus. Front. Horm. Res. 2018, 50, 161–176. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; on behalf of Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ehrampoush, E.; Mirzay Razzaz, J.; Arjmand, H.; Ghaemi, A.; Raeisi Shahraki, H.; Ebrahim Babaei, A.; Osati, S.; Homayounfar, R. The association of vitamin D levels and insulin resistance. Clin. Nutr. ESPEN 2021, 42, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Sliwinska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E. Vitamin D deficiency in the aetiology of obesity-related insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3146. [Google Scholar] [CrossRef]
- Cefalo, C.M.A.; Conte, C.; Sorice, G.P.; Moffa, S.; Sun, V.A.; Cinti, F.; Salomone, E.; Muscogiuri, G.; Brocchi, A.A.G.; Pontecorvi, A.; et al. Effect of Vitamin D Supplementation on Obesity-Induced Insulin Resistance: A Double-Blind, Randomized, Placebo-Controlled Trial. Obesity 2018, 26, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of Vitamin D Supplementation on Glycemic Control in Prediabetes: A Meta-Analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Gunton, J.E.; Girgis, C.M.; Baldock, P.A.; Lips, P. Bone muscle interactions and vitamin D. Bone 2015, 80, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Brennan-Speranza, T.C.; Fraser, D.R. Is it reasonable to ignore vitamin D status for musculoskeletal health? Fac. Rev. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Cell Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef]
- Teixeira, M.A.; De Feudis, M.; Reano, S.; Raiteri, T.; Scircoli, A.; Zaggia, I.; Ruga, S.; Salvadori, L.; Prodam, F.; Marzullo, P.; et al. Cholecalciferol (vitamin D3) has a direct protective activity against interleukin 6-induced atrophy in C2C12 myotubes. Aging 2021, 13, 4895–4910. [Google Scholar] [CrossRef]
- Romeu Montenegro, K.; Carlessi, R.; Cruzat, V.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kondo, Y.; Fukuda, T.; Kitagawa, N.; Okada, H.; et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients 2021, 13, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Baek, K.H.; Song, K.H.; Il Kang, M.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: The Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab. 2011, 96, 3250–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.A.; Cho, H.; Eun, C.R.; Yoo, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Park, M.H.; Han, C.; et al. Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: The Ansan Geriatric Study. J. Am. Geriatr. Soc. 2012, 60, 700–706. [Google Scholar] [CrossRef]
- Oh, C.; Jeon, B.H.; Reid Storm, S.N.; Jho, S.; No, J.K. The most effective factors to offset sarcopenia and obesity in the older Korean: Physical activity, vitamin D, and protein intake. Nutrition 2017, 33, 169–173. [Google Scholar] [CrossRef]
- Manoy, P.; Anomasiri, W.; Yuktanandana, P.; Tanavalee, A.; Ngarmukos, S.; Tanpowpong, T.; Honsawek, S. Elevated serum leptin levels are associated with low vitamin D, sarcopenic obesity, poor muscle strength, and physical performance in knee osteoarthritis. Biomarkers 2017, 22, 723–730. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Lee, O. Association of serum vitamin D with osteosarcopenic obesity: Korea National Health and Nutrition Examination Survey 2008–2010. J. Cachexia Sarcopenia Muscle 2017, 8, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Bislev, L.S.; Grove-Laugesen, D.; Rejnmark, L. Vitamin D and Muscle Health: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials. J. Bone Miner. Res. 2021, 36, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, in press. [Google Scholar] [CrossRef]
- Jabbour, J.; Rahme, M.; Mahfoud, Z.R.; El-Hajj Fuleihan, G. Effect of high dose vitamin D supplementation on indices of sarcopenia and obesity assessed by DXA among older adults: A randomized controlled trial. Endocrine 2022, 76, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in Vitamin D: Summary Statement From an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, S69–S77. [Google Scholar] [CrossRef]
- Ilich, J.Z. Osteosarcopenic adiposity syndrome update and the role of associated minerals and vitamins. Proc. Nutr. Soc. 2021, 80, 344–355. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Parrinello, E.; Barazzoni, R.; Busetto, L.; Donini, L.M. Therapeutic strategies for sarcopenic obesity: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 33–41. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Styner, M.; Uzer, G.; Patel, V.S.; Wright, L.E.; Ness, K.K.; Guise, T.A.; Rubin, J.; Rubin, C.T. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 2019, 15, 339–355. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. https://doi.org/10.3390/nu14091816
Di Filippo L, De Lorenzo R, Giustina A, Rovere-Querini P, Conte C. Vitamin D in Osteosarcopenic Obesity. Nutrients. 2022; 14(9):1816. https://doi.org/10.3390/nu14091816
Chicago/Turabian StyleDi Filippo, Luigi, Rebecca De Lorenzo, Andrea Giustina, Patrizia Rovere-Querini, and Caterina Conte. 2022. "Vitamin D in Osteosarcopenic Obesity" Nutrients 14, no. 9: 1816. https://doi.org/10.3390/nu14091816