Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil
Abstract
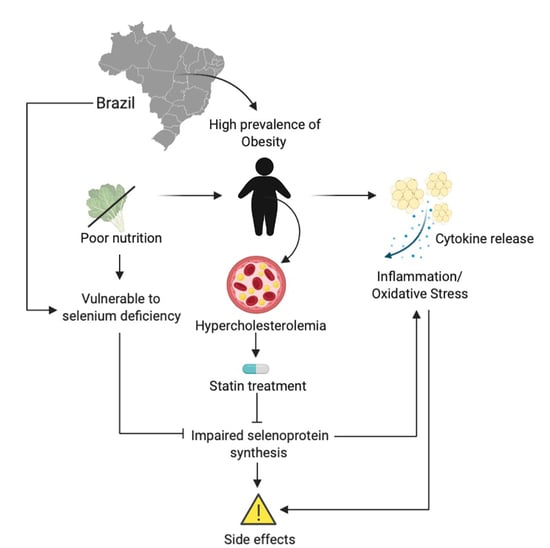
:1. Introduction
2. Obesity: Overfed but Undernourished
2.1. Nutritional Aspects of the Obesogenic Environment in Brazil
2.2. Dietary Patterns of the Brazilian Population
2.3. Nutrient Deficiency
3. Selenium and Selenoproteins
3.1. Disruptions in Selenoprotein Synthesis
3.2. Selenium Intake and Status of the Brazilian Population
4. Intersection between Dietary Selenium and Obesity in Brazil
5. Statins and Associated Side Effects: Occurrences in the Brazilian Population
5.1. Lipid Dysregulation in Obesity
5.2. Statin Use in Brazil
5.3. Statin-Associated Side Effects among Brazilians
5.4. Selenium Supplementation and SAMS in Brazil
6. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vecchié, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Frühbeck, G.; Montecucco, F. Obesity Phenotypes and Their Paradoxical Association with Cardiovascular Diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Qasim, A.; Turcotte, M.; de Souza, R.J.; Samaan, M.C.; Champredon, D.; Dushoff, J.; Speakman, J.R.; Meyre, D. On the Origin of Obesity: Identifying the Biological, Environmental and Cultural Drivers of Genetic Risk among Human Populations. Obes. Rev. 2018, 19, 121–149. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- WHO Fact Sheet: Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 April 2021).
- IBGE. Pesquisa Nacional de Saúde 2019: Informações Sobre Domicílios, Acesso e Utilização Dos Serviços de Saúde. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101748.pdf (accessed on 2 April 2021).
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Bahia, L.; Coutinho, E.S.F.; Barufaldi, L.A.; De Azevedo Abreu, G.; Malhão, T.A.; Ribeiro De Souza, C.P.; Araujo, D.V. The Costs of Overweight and Obesity-Related Diseases in the Brazilian Public Health System: Cross-Sectional Study. BMC Public Health 2012, 12, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, M.L.; Santos, L.M.P.; Silvada, E.N. Direct Healthcare Cost of Obesity in Brazil: An Application of the Cost-of-Illness Method from the Perspective of the Public Health System in 2011. PLoS ONE 2015, 10, e0121160. [Google Scholar] [CrossRef] [Green Version]
- Rtveladze, K.; Marsh, T.; Webber, L.; Kilpi, F.; Levy, D.; Conde, W.; McPherson, K.; Brown, M. Health and Economic Burden of Obesity in Brazil. PLoS ONE 2013, 8, e68785. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why Primary Obesity Is a Disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- dos Passos, C.M.; Maia, E.G.; Levy, R.B.; Martins, A.P.B.; Claro, R.M. Association between the Price of Ultra-Processed Foods and Obesity in Brazil. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Won, K.B.; Hur, S.H.; Nam, C.W.; Ann, S.H.; Park, G.M.; Lee, S.G.; Kim, H.E.; Cho, Y.K.; Yoon, H.J.; Park, H.S.; et al. Evaluation of the Impact of Statin Therapy on the Obesity Paradox in Patients with Acute Myocardial Infarction. Medicine (United States) 2017, 9, e7180. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, E.D.; Engin, A. Two Important Controversial Risk Factors in SARS-CoV-2 Infection: Obesity and Smoking. Environ. Toxicol. Pharmacol. 2020, 78, 103411. [Google Scholar] [CrossRef]
- WHO. Noncommunicable Diseases. Who 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 2 April 2021).
- McCafferty, B.J.; Hill, J.O.; Gunn, A.J. Obesity: Scope, Lifestyle Interventions, and Medical Management. Tech. Vasc. Interv. Radiol. 2020, 23, 100653. [Google Scholar] [CrossRef]
- Castillo, J.J.; Orlando, R.A.; Garver, W.S. Gene-Nutrient Interactions and Susceptibility to Human Obesity. Genes Nutr. 2017, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and Epigenetics in Obesity. Metab. Clin. Exp. 2019, 92, 37–50. [Google Scholar] [CrossRef] [Green Version]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of Macronutrients to Obesity: Implications for Precision Nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Thomas-Valdés, S.; das Graças V Tostes, M.; Anunciação, P.C.; da Silva, B.P.; Sant’Ana, H.M.P. Association between Vitamin Deficiency and Metabolic Disorders Related to Obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 3332–3343. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and Dyslipidemia. Metab. Clin. Exp. 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity: Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, D.; Schaeffeler, E.; Schwab, M.; Mörike, K. Mechanisms and Assessment of Statin-Related Muscular Adverse Effects. Br. J. Clin. Pharmacol. 2014, 78, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Alvarado-Cardenas, M.; Pinal-Fernández, I.; Trallero-Araguás, E.; Milisenda, J.C.; Martínez, M.Á.; Marín, A.; Labrador-Horrillo, M.; Juárez, C.; Grau-Junyent, J.M. Statin-Induced Myalgia and Myositis: An Update on Pathogenesis and Clinical Recommendations. Expert Rev. Clin. Immunol. 2018, 14, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Wierzbicki, A. Statins, Muscle Disease and Mitochondria. J. Clin. Med. 2017, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Bubenik, J.L.; Miniard, A.C.; Driscoll, D.M. Characterization of the UGA-Recoding and SECIS-Binding Activities of SECIS-Binding Protein 2. RNA Biol. 2014, 11, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Kromer, A.; Moosmann, B. Statin-Induced Liver Injury Involves Cross-Talk between Cholesterol and Selenoprotein Biosynthetic Pathways. Mol. Pharmacol. 2009, 75, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Moosmann, B.; Behl, C. Selenoproteins, Cholesterol-Lowering Drugs, and the Consequences: Revisiting of the Mevalonate Pathway. Trends Cardiovasc. Med. 2004, 14, 273–281. [Google Scholar] [CrossRef]
- Grover, H.S.; Luthra, S.; Maroo, S. Are Statins Really Wonder Drugs? J. Formos. Med Assoc. 2014, 113, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosmann, B.; Behl, C. Selenoprotein Synthesis and Side-Effects of Statins. Lancet 2004, 363, 892–894. [Google Scholar] [CrossRef]
- Warner, G.J.; Berry, M.J.; Moustafa, M.E.; Carlson, B.A.; Hatfield, D.L.; Faust, J.R. Inhibition of Selenoprotein Synthesis by Selenocysteine TRNA([Ser]Sec) Lacking Isopentenyladenosine. J. Biol. Chem. 2000, 275, 28110–28119. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil Nuts: Nutritional Composition, Health Benefits and Safety Aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Gomes, J.C.; Bellato, C.R.; Jordão, C.P. Concentrações de Selênio Em Alimentos Consumidos No Brasil. Rev. Panam. De Salud Publica Pan Am. J. Public Health 2002, 11, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Almondes, K.G.S.; Cardoso, B.R.; Cominetti, C.; Nogueira, N.N.; Marreiro, D.N.; Oliveira, T.F.; Loureiro, A.P.M.; Cozzolino, S.M.F. The Redox Balance of Healthy Brazilian Adults Is Associated with GPX1 Pro198Leu and -602A/G Polymorphisms, Selenium Status, and Anthropometric and Lifestyle Parameters. Food Funct. 2018, 9, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Rita Cardoso, B.; Apolinário, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Effects of Brazil Nut Consumption on Selenium Status and Cognitive Performance in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Pilot Trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium Behavior in the Soil Environment and Its Implication for Human Health. Ciência E Agrotecnol. 2017, 41, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376. [Google Scholar] [CrossRef]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- da Costa Louzada, M.L.; Ricardo, C.Z.; Steele, E.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. The Share of Ultra-Processed Foods Determines the Overall Nutritional Quality of Diets in Brazil. Public Health Nutr. 2018, 21, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Monteiro, C.; Cannon, G.; Levy, R.B.; Moubara, J.-C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The Star Shines Bright. Position Paper 2 (PDF Download Available). World Nutr. 2016, 7, 1–3. [Google Scholar]
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The Share of Ultra-Processed Foods and the Overall Nutritional Quality of Diets in the US: Evidence from a Nationally Representative Cross-Sectional Study. Popul. Health Metr. 2017, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Laura Da Costa Louzada, M.; Machado, P.P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. 2019. Available online: http://www.fao.org/3/ca5644en/ca5644en.pdf (accessed on 2 April 2019).
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- da Oliveira, M.S.S.; da Santos, L.A.S. Dietary Guidelines for Brazilian Population: An Analysis from the Cultural and Social Dimensions of Food. Cienc. E Saude Coletiva 2020, 2519–2528. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.R.; Cannon, G. Increasing Consumption of Ultra-Processed Foods and Likely Impact on Human Health: Evidence from Brazil. Public Health Nutr. 2011, 14, 5–13. [Google Scholar] [CrossRef]
- Mohapatra, S.; Gangadharan, K.; Pitchumoni, C.S. Malnutrition in Obesity before and after Bariatric Surgery. Dis. A Mon. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, S.M.F. Deficiências de Minerais. Estud. Av. 2007, 21, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Jameson, R.R.; Diamond, A.M. A Regulatory Role for Sec TRNA[Ser]Sec in Selenoprotein Synthesis. RNA 2004, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [Green Version]
- Lescure, A.; Fagegaltier, D.; Carbon, P.; Krol, A. Protein Factors Mediating Selenoprotein Synthesis. Curr. Protein Pept. Sci. 2005, 3, 143–151. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Tobe, R.; Mihara, H. Delivery of Selenium to Selenophosphate Synthetase for Selenoprotein Biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Carlson, B.A.; Zhang, Y.; Mix, H.; Kryukov, G.V.; Glass, R.S.; Berry, M.J.; Gladyshev, V.N.; Hatfield, D.L. New Developments in Selenium Biochemistry: Selenocysteine Biosynthesis in Eukaryotes and Archaea. Proc. Biol. Trace Elem. Res. 2007, 119, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, M.T.; Moyle, M.W.; Aggarwal, G.; Carlson, B.A.; Anderson, C.B. A Recoding Element That Stimulates Decoding of UGA Codons by Sec TRNA [Ser]Sec. RNA 2007, 13, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seale, L.A. Selenocysteine β-Lyase: Biochemistry, Regulation and Physiological Role of the Selenocysteine Decomposition Enzyme. Antioxidants 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, B.A.; Xu, X.M.; Kryukov, G.V.; Rao, M.; Berry, M.J.; Gladyshev, V.N.; Hatfield, D.L. Identification and Characterization of Phosphoseryl-TRNA[Ser]Sec Kinase. Proc. Natl. Acad. Sci. USA 2004, 12848–12853. [Google Scholar] [CrossRef] [Green Version]
- Carlson, B.A.; Yoo, M.H.; Tsuji, P.A.; Gladyshev, V.N.; Hatfield, D.L. Mouse Models Targeting Selenocysteine TRNA Expression for Elucidating the Role of Selenoproteins in Health and Development. Molecules 2009, 14, 3509–3527. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, M.F.; Barra, G.B.; Naves, L.A.; Velasco, L.F.R.; Castro, P.G.G.; de Castro, L.C.G.; Amato, A.A.; Miniard, A.; Driscoll, D.; Schomburg, L.; et al. Selenoprotein-Related Disease in a Young Girl Caused by Nonsense Mutations in the SBP2 Gene. J. Clin. Endocrinol. Metab. 2010, 95. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Dumitrescu, A.M. Inherited Defects in Thyroid Hormone Cell-Membrane Transport and Metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Seale, L.A.; Hashimoto, A.C.; Kurokawa, S.; Gilman, C.L.; Seyedali, A.; Bellinger, F.P.; Raman, A.V.; Berry, M.J. Disruption of the Selenocysteine Lyase-Mediated Selenium Recycling Pathway Leads to Metabolic Syndrome in Mice. Mol. Cell. Biol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, L.M.; Hashimoto, A.C.; Torres, D.J.; Berry, M.J.; Seale, L.A. Effects of Selenium Supplementation on Diet-Induced Obesity in Mice with a Disruption of the Selenocysteine Lyase Gene. J. Trace Elem. Med. Biol. 2020, 62, 126596. [Google Scholar] [CrossRef] [PubMed]
- Seale, L.A.; Ha, H.Y.; Hashimoto, A.C.; Berry, M.J. Relationship between Selenoprotein P and Selenocysteine Lyase: Insights into Selenium Metabolism. Free Radic. Biol. Med. 2018, 127, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.M.; Hashimoto, A.C.; Torres, D.J.; Alfulaij, N.; Peres, R.; Sultana, R.; Maunakea, A.K.; Berry, M.J.; Seale, L.A. Effect of Statin Treatment in Obese Selenium-Supplemented Mice Lacking Selenocysteine Lyase. Mol. Cell. Endocrinol. 2021, 533, 111335. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, A.A.; Castilho, I.N.B.; Welz, B.; Carasek, E.; Gonzaga Martens, I.B.; Martens, A.; Cozzolino, S.M.F. Method Development and Optimization for the Determination of Selenium in Bean and Soil Samples Using Hydride Generation Electrothermal Atomic Absorption Spectrometry. Talanta 2011, 85, 1350–1356. [Google Scholar] [CrossRef]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil Nuts: An Effective Way to Improve Selenium Status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, L.F.M.F.; Stockler-Pinto, M.B.; Mafra, D. Brazil Nut Consumption Modulates Nrf2 Expression in Hemodialysis Patients: A Pilot Study. Mol. Nutr. Food Res. 2016, 60, 1719–1724. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids 2000. In Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds; National Academies Press: Washington, DC, USA, 2000. [CrossRef]
- Sebastian, R.S.; Enns, C.W.; Goldman, J.D.; Moshfegh, A.J. Trends in Food Intakes of U.S. Adults: Findings from the Continuing Survey of Food Intakes by Individuals 1994–1996 and What We Eat in America, NHANES 2001–2002 and 2009–2010. FASEB J. 2013, 27, 848. [Google Scholar] [CrossRef]
- Cominetti, C.; Duarte, G.; Cozzolino, S. Selênio, in Funções Plenamente Reconhecidas de Nutrientes; International Life Sciences Institute do Brasil: São Paulo, Brazil, 2017; Available online: https://ilsi.org/brasil/wp-content/uploads/sites/9/2016/05/08-Selênio.pdf (accessed on 2 April 2021).
- Hosseini, B.; Saedisomeolia, A.; Allman-Farinelli, M. Association Between Antioxidant Intake/Status and Obesity: A Systematic Review of Observational Studies. Biol. Trace Elem. Res. 2017, 175, 287–297. [Google Scholar] [CrossRef]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between Body Mass Index and the Prevalence of Low Micronutrient Levels among US Adults. Medgenmed Medscape Gen. Med. 2006, 8, 59. [Google Scholar]
- Arnaud, J.; Bertrais, S.; Roussel, A.M.; Arnault, N.; Ruffieux, D.; Favier, A.; Berthelin, S.; Estaquio, C.; Galan, P.; Czernichow, S.; et al. Serum Selenium Determinants in French Adults: The SU.VI.M.AX Study. Br. J. Nutr. 2006, 95, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Tinkov, A.A.; Skalnaya, M.G.; Ajsuvakova, O.P.; Serebryansky, E.P.; Chao, J.C.J.; Aschner, M.; Skalny, A.V. Selenium, Zinc, Chromium, and Vanadium Levels in Serum, Hair, and Urine Samples of Obese Adults Assessed by Inductively Coupled Plasma Mass Spectrometry. Biol. Trace Elem. Res. 2021, 199, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Soares de Oliveira, A.R.; Jayanne Clímaco Cruz, K.; Beatriz Silva Morais, J.; Rocha Dos Santos, L.; Rodrigues de Sousa Melo, S.; Fontenelle, L.C.; Santos de Sousa, G.; Costa Maia, C.S.; Oliveira Duarte de Araújo, C.; Leal Mendes, I.; et al. Selenium Status and Oxidative Stress in Obese: Influence of Adiposity. Eur. J. Clin. Investig. 2021, e13538. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; Feitosa, M.M.; Freitas, T.E.C.; Severo, J.S.; Morais, J.B.S.; Henriques, G.S.; Oliveira, F.E.; Moita Neto, J.M.; do Nascimento Marreiro, D. Selenium Status and Its Relationship with Thyroid Hormones in Obese Women. Clin. Nutr. ESPEN 2021, 41, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Botelho, A.; Queiroz Gurgel, R.; Simeone Henriques, G.; dos Santos, C.B.; Afonso Jordão, A.; Nascimento Faro, F.; Silveira Souto, F.M.; Rodrigues Santos, A.P.; Eduardo Cuevas, L. Micronutrient Deficiencies in Normal and Overweight Infants in a Low Socio-Economic Population in North-East Brazil. Paediatr. Int. Child Health 2016, 36, 198–202. [Google Scholar] [CrossRef]
- Retondario, A.; de Moura Souza, A.; Fernandes, R.; Bricarello, L.P.; de Almeida Alves, M.; Zeni, L.A.Z.R.; de Moraes Trindade, E.B.S.; de Assis Guedes de Vasconcelos, F. Usual Intake and Dietary Sources of Selenium in Adolescents: A Cross-Sectional School-Based Study. Clin. Nutr. ESPEN 2019, 33, 91–97. [Google Scholar] [CrossRef]
- Cecília Thé Maia de Arruda Falcão, R.; de Oliveira Lyra, C.; Márcia Medeiros de Morais, C.; Galvão Bacurau Pinheiro, L.; Fátima Campos Pedrosa, L.; Carla Vieira Cunha Lima, S.; Cavalcanti Maurício Sena-Evangelista, K. Processed and Ultra-Processed Foods Are Associated with High Prevalence of Inadequate Selenium Intake and Low Prevalence of Vitamin B1 and Zinc Inadequacy in Adolescents from Public Schools in an Urban Area of Northeastern Brazil. PLoS ONE 2019, 14, e0224984. [Google Scholar] [CrossRef]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-Analysis of 43 Observational Studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Stenzel, A.P.; Carvalho, R.; Jesus, P.; Bull, A.; Pereira, S.; Saboya, C.; Ramalho, A. Serum Antioxidant Associations with Metabolic Characteristics in Metabolically Healthy and Unhealthy Adolescents with Severe Obesity: An Observational Study. Nutrients 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- da Cunha, S.; Albanesi Filho, F.M.; Antelo, D.S.; de Souza, M.M. Serum Sample Levels of Selenium and Copper in Healthy Volunteers Living in Rio de Janeiro City. Sci. Total Environ. 2003, 301, 51–54. [Google Scholar] [CrossRef]
- Cerqueira, N.M.F.S.A.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef] [PubMed]
- Karr, S. Epidemiology and Management of Hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- Gus, I.; Ribeiro, R.A.; Kato, S.; Bastos, J.; Medina, C.; Zazlavsky, C.; Portal, V.L.; Timmers, R.; Markoski, M.M.; Gottschall, C.A.M. Variations in the Prevalence of Risk Factors for Coronary Artery Disease in Rio Grande Do Sul-Brazil: A Comparative Analysis between 2002 and 2014. Arq. Bras. Cardiol. 2015, 105, 573–579. [Google Scholar] [CrossRef]
- Garcez, M.R.; Pereira, J.L.; de Mello Fontanelli, M.; Marchioni, D.M.L.; Fisberg, R.M. Prevalence of Dyslipidemia According to the Nutritional Status in a Representative Sample of São Paulo. Arq. Bras. Cardiol. 2014, 103, 476–484. [Google Scholar] [CrossRef]
- Malta, D.C.; Teixeira, R.; de Oliveira, G.M.M.; Ribeiro, A.L. Mortalidade Por Doenças Cardiovasculares Segundo o Sistema de Informação Sobre Mortalidade e as Estimativas Do Estudo Carga Global de Doenças No Brasil, 2000–2017. Arq. Bras. Cardiol. 2020, 115, 152–160. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and Its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krähenbühl, S. Mechanisms of Statin-Associated Skeletal Muscle-Associated Symptoms. Pharmacol. Res. 2020, 104201. [Google Scholar] [CrossRef]
- do Nascimento, R.C.R.M.; Guerra, A.A.; Alvares, J.; Gomes, I.C.; Godman, B.; Bennie, M.; Kurdi, A.B.; de Acurcio, F.A. Statin Use in Brazil: Findings and Implications. Curr. Med. Res. Opin. 2018, 34, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, L.M.; Fernandes de Lima, L.; Ferraz-Bannitz, R.; Takaara, D.; Coimbra Romano, B.; Braga Costa, T.M.; Foss de Freitas, M.C.; Bueno, A.C.; Barbosa Júnior, F.; Marliere Navarro, A. Association between Creatine Kinase Activity, Oxidative Stress and Selenoproteins MRNA Expression Changes after Brazil Nut Consumption of Patients Using Statins. Clin. Nutr. 2020, 39, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Li, L.; Lu, D.; Yazdanparast, A.; Wang, L.; Kreutz, R.P.; Whipple, E.C.; Schleyer, T.K. A Comprehensive Review and Meta-Analysis of Risk Factors for Statin-Induced Myopathy. Eur. J. Clin. Pharmacol. 2018, 74, 1099–1109. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, P.S.; Huang, Y.H.; Tang, S.C.; Li, Y.H.; Yeh, H.I. 2019 Taiwan Society of Lipids and Atherosclerosis Expert Consensus Statement on Statin Intolerance. J. Formos. Med Assoc. 2019, 118, 1385–1392. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. Safety and Efficacy of Statin Therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef]
- Castro, P.F.; Ribeiro, E.; Dorea, E.L.; Pinto, G.A.; Hirata, R.D.C. Factors Associated with Statin-Related Adverse Muscular Events in Adult Dyslipidemic Outpatients. Braz. J. Pharm. Sci. 2017, 53, e00199. [Google Scholar] [CrossRef] [Green Version]
- Kristjansson, R.P.; Oddsson, A.; Helgason, H.; Sveinbjornsson, G.; Arnadottir, G.A.; Jensson, B.O.; Jonasdottir, A.; Jonasdottir, A.; Bragi Walters, G.; Sulem, G.; et al. Common and Rare Variants Associating with Serum Levels of Creatine Kinase and Lactate Dehydrogenase. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Balestrino; Adriano Creatine as a Candidate to Prevent Statin Myopathy. Biomolecules 2019, 9, 496. [CrossRef] [PubMed] [Green Version]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Adverse Effects of Statins—Mechanisms and Consequences. Curr. Drug Saf. 2009, 4, 209–228. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maranhão, P.A.; Kraemer-Aguiar, L.G.; de Oliveira, C.L.; Kuschnir, M.C.C.; Vieira, Y.R.; Souza, M.G.C.; Koury, J.C.; Bouskela, E. Brazil Nuts Intake Improves Lipid Profile, Oxidative Stress and Microvascular Function in Obese Adolescents: A Randomized Controlled Trial. Nutr. Metab. 2011, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cominetti, C.; de Bortoli, M.C.; Garrido, A.B.; Cozzolino, S.M.F. Brazilian Nut Consumption Improves Selenium Status and Glutathione Peroxidase Activity and Reduces Atherogenic Risk in Obese Women. Nutr. Res. 2012, 32, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.M.; Bueno, A.C.; de Lima, L.F.; Ferraz-Bannitz, R.; Dessordi, R.; Guimarães, M.P.; de Freitas, M.C.F.; Barbosa, F.; Navarro, A.M. Genetically-Determined Variations of SELENOP Are Associated with Antioxidant, Muscular, and Lipid Biomarkers in Response to Brazil Nut Consumption by Patients Using Statins. Br. J. Nutr. 2021, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Reference | Study Population | Country | Conclusion |
|---|---|---|---|
| Galan et al., 2005 in Hosseini et al., 2017 (review) [78] | 3128 adults (58 % women) | French | No association between zinc and selenium concentration and anthropometric data |
| Tascilar et al., 2011 in Hosseini et al., 2017 (review) [78] | 34 with obesity and 33 healthy children | Turkey | No differences in serum trace element levels (selenium and zinc) between subjects with obesity and healthy ones |
| Kimmons et al., 2006 [79] | 16,181 adults | United States of America | Lower serum selenium was associated with overweight and obesity, both in men and premenopausal women |
| Arnaud et al., 2006 [80] | 13,017 subjects (7876 women and 5141 men) | French | Obesity was associated with decreased serum selenium levels only in women |
| Tinkov et al., 2021 [81] | 395 adults (199 lean and 196 with obesity) | Russia | High serum selenium was associated with obesity prevalence, particularly in hypertensive individuals |
| Soares de Oliveira et al., 2021 [82] | 139 women (63 with obesity, and 76 normal weight) | Brazil | Plasma selenium concentration was negatively associated with obesity and visceral adiposity. Women with obesity reduced plasma and erythrocyte and increased urinary excretion of selenium. |
| Fontanelle et al., 2020 [83] | 69 euthyroid women (35 with obesity and 34 normal weight) | Brazil | Negative association between plasma selenium and obesity, with higher clearance through excretion but not higher selenium levels in the urine. |
| Jardim-Botelho et al., 2016 [84] | 153 infants aged 2–11 months | Brazil | 91% of subjects with selenium deficiency |
| Retondario et al., 2019 [85] | 76,957 adolescents aged from 12–17 years (49.7% girls) | Brazil | Prevalence of adequate selenium intake |
| Falcão et al., 2019 [86] | 444 adolescents | Brazil | Intake of ultra-processed foods was correlated with inadequate selenium intake |
| Lane et al., 2021 [87] | 891,723 subjects | - | Meta-analysis showed consumption of ultra-processed food was associated with increased risk of overweight |
| Stenzen et al., 2018 [88] | 60 adolescents with severe obesity | Brazil | 36% of selenium-deficient subjects based on serum selenium levels |
| Da Cunha et al., 2003 [89] | 30 healthy adults | Brazil | Prevalence of mild selenium deficiency, particularly among men. No association between selenium and obesity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, L.M.; Navarro, A.M.; Seale, L.A. Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil. Nutrients 2021, 13, 2027. https://doi.org/10.3390/nu13062027
Watanabe LM, Navarro AM, Seale LA. Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil. Nutrients. 2021; 13(6):2027. https://doi.org/10.3390/nu13062027
Chicago/Turabian StyleWatanabe, Ligia M., Anderson M. Navarro, and Lucia A. Seale. 2021. "Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil" Nutrients 13, no. 6: 2027. https://doi.org/10.3390/nu13062027