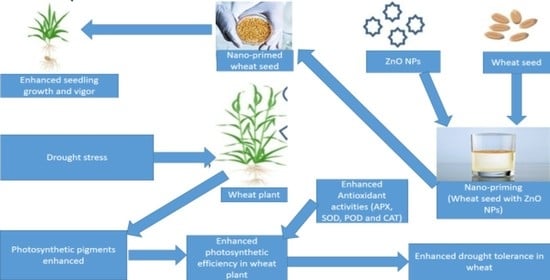
1. Introduction
Globally, wheat (
Triticum aestivum L.) is an important staple food for millions of people and is being cultivated in all inhabitable continents [
1,
2]. Due to climate change and the increasing food demand, improving wheat yield per unit area has become pressing [
3,
4]. It is projected that if the current changes in the climate continue, then wheat production may decline by over 50% in next two decades [
5,
6]. To address these challenges, the use of nanotechnology for developing nanoparticles (NPs) to enhance plant growth and yield under stressful conditions hold potential and bright prospects [
7,
8]. Additionally, the application of NPs for boosting wheat yield through the amelioration of drought’s deleterious impacts might play a significant role in addressing the food security concerns of the modern era [
6,
9,
10]. Recently, many NP products such as nano-fertilizers, nano-pesticides, and nano-sensors have already been tested and employed to enhance crops productivity [
11,
12]. The NPs help to increase the nutrient-use efficiency of plants. Because of their small size, NPs cover a larger surface area and directly improve the physiological functions of crop plants [
11]. Various methods have been used to supply NPs to plants such as seed coating, soil application and foliar spray under a stressful environment. The application of NPs through seed priming (SP) is an innovative technique that may improve seed vigor, which is a prerequisite of better stand establishment under normal or even stressful environments [
12]. In the SP method, seeds are soaked in water (hydro-priming) and aerated solutions (osmo-priming) for a specific time period that can trigger the metabolic processes (MPs). These MPs are generally activated during the early phase of germination (pre-germinate metabolism) and therefore increase the rate of germination and seedling establishment [
13,
14]. Different priming solutions (polyethylene glycol, hormones, nutrients, organic salts, etc.) help the plants to off-set the adverse effects of abiotic stresses including DS [
15,
16]. For the SP, different NPs such as gold (Au), silver (Ag), iron (Fe), copper (Cu), zinc oxide (ZnO), zinc (Zn), carbon-based NPs fullerene and carbon nanotubes have been used to mitigate the harmful effects of DS [
15,
17].
There are research and knowledge gaps regarding the optimum source and dose of NPs applied as SP (seed priming) agents because different NPs tend to use atypical mechanisms for mitigating the adverse effects of DS. The studies of nano-priming effects on seed germination, early stand establishment and plant growth in wheat under normal and stressful conditions have not been elucidated so far. Zinc (Zn) is an essential micronutrient required for plant development and growth as it is an essential component of over 300 proteins and enzymes synthesized by plants under normal and scant water conditions [
16,
18]. Zn is needed for a variety of physiological functions such as pollination, growth regulation, antioxidant function and protein synthesis. Moreover, it also plays a vital role in photosynthesis, maintenance of cell membranes, detoxification of free radicals and gene expression under water-deficient conditions [
19,
20,
21].
The primary challenge of Zn application as ZnO is the low solubility of Zn in soil and higher losses to terrestrial ecosystems [
22,
23,
24]. However, NPs of ZnO might overcome these problems due to their high solubility, availability and reactivity within plant tissues [
25]. Additionally, these have drawn the attention of researchers owing to their unique photo-oxidizing, physiological and biochemical capacity, as well as their unique functions in plants physiological functions [
6,
26]. The SP with NPs of ZnO significantly increased the Zn content in the primed seeds, leading to improved seedling vigor, growth and economic yield [
6,
26,
27]. Likewise, Tului et al. [
28] inferred that ZnO NPs had positive effects on the growth of chickpeas (
Cicer arientinum), while their growth-promoting impact has also been recorded for mung bean [
29], cucumber [
30]), alfalfa [
31] and tomato [
32]. However, previous studies present contrasting findings pertaining to the most superior and effective dose of ZnO NPs for ameliorating the adverse effects of DS, while considerable research gaps also exist regarding their efficiency for different genotypes of wheat. Thus, the research hypothesis of this study is that wheat genotypes might respond differently to varying doses of ZnO NPs under atypical DS levels. Therefore, this study aims to evaluate wheat cultivars response to different doses of ZnO nanoparticles in terms of numerous botanical, physiological and biochemical traits, while the ultimate goal was to identify the drought resistant wheat cultivar and the most superior dose of ZnO NPs for ameliorating the adverse effects of DS under changing climate scenarios.
3. Results
The results show that DS reduces the morphological parameters such as the shoot and root lengths of both wheat varieties, however, SP with ZnO NPs significantly enhances the morphological parameters compared with untreated seeds. Seed priming with ZnO NPs significantly increases the shoot and root lengths (
Table 1) as the maximum shoot (69.34 cm) and root length (23.56 cm) are measured for Zincol-16 when the ZnO NPs (120 ppm) is applied under well-watered (100% FC) conditions. Similarly, seeds of Ujala-16 primed with 120 ppm of ZnO NPs and grown under 100% FC show higher shoot (63.20 cm) and root length (21.47 cm) as compared with 160 ppm of ZnO NPs (58.95 cm shoot length and 20.04 cm root length). Zincol-16 is more responsive to ZnO NPs as compared with Ujala-16 under well-watered (100% FC) and water-stressed (50% FC) conditions. Wheat plants from untreated seeds record the minimum shoot (24.36 to 29.89 cm) and root length (8.27 to 10.15 cm in Ujala-16 and Zincole-16, respectively). Similarly, shoot and root fresh and dry weights significantly increase with seed priming with ZnO NPs. All of the ZnO NPs treatments increase shoot and root dry weights as compared with untreated seeds. The maximum shoot fresh (13.26 g) and dry weight (5.45 g) is recorded in Zincol-16 when primed with ZnO NPs at 120 ppm and grown at 100% FC, followed by the same wheat variety at 160 ppm and Ujala-16 primed with 120 ppm of ZnO NPs. The DS decreases the shoot fresh and dry weights but the maximum reduction in shoot fresh (4.66 g) and root dry weight (1.91 g) are recorded for Ujala-16 grown without seed treatment.
Priming of wheat seed with ZnO NPs results in significantly higher leaf area (LA) per plants as compared with untreated seeds (
Table 2). The Zincol-16 cultivar exhibits the maximum LA (417 cm
2) when seeds are primed with ZnO NPs (120 ppm) and sown under 100% FC as compared to Ujala-16 (318 cm
2). The leaf water potential represents a useful index of soil water stress and provide insights regarding plant-water relationships. Data shows that wheat plants under DS show a higher leaf water potential. Plants grown from untreated seeds show higher water potential. In plant leaf water potential, the improvement is shown after the treatment of ZnO NPs, and minimum leaf water potential (−24.00 bars) is recorded for Ujala-16 grown subjected to SP with ZnO NPs (160 ppm) under 100% FC, followed by Zincol-16 (−31.66 bars). Likewise, the relative water content (RWC) increases with SP with ZnO NPs (
Table 2) as the maximum RWC is observed for Zincol-16 (82.66%) in response to ZnO NPs applied at the rate of 120 ppm under 100% FC, which is on par with Ujala-16 treated seeds with ZnO NPs at 160 ppm (82.00%). Both wheat varieties show minimum RWC when seeds are not primed and grown in water stress conditions (50% FC), however, the lowest RWC (62.66%) is noted for Zincol-16. Wheat plants show a higher content of Chl a, b and total Chl content when seeds are primed with ZnO NPs. All the treatments of ZnO NPs improve Chl
a,
b and total Chl over the plants grown from untreated seeds (
Table 3). The DS (50% FC) reduce the Chl
a,
b and total Chl as compared with well-watered conditions (100% FC). The maximum Chl
a (1.69 mg/g FW),
b (0.71 mg/g FW) and total Chl (2.46 mg/g FW) contents are recorded for Zincol-16 under ZnO NPs applied at 120 ppm with 100% FC, which is at par with Ujala-16 primed with ZnO NPs at 160 ppm. The lowest Chl
a (1.33 mg/g FW),
b (0.43 mg/g FW), and total Chl (1.76 mg/g FW) content is recorded for Zincol-16 when seeds are sown under drought-stressed conditions without ZnO treatment followed by Ujala-16.
Interestingly, the untreated seeds of Ujala-16 under DS conditions show higher proline content. ZnO NPs also significantly increase the proline content of wheat. The maximum proline content (5.13 µmoles g−1 FW) is recorded for Zincol-16 when seeds are primed with ZnO NPs at 120 ppm and grown under 50% FC level, followed by Zincol-16 at 120 ppm (4.74 µmoles g−1 FW) and Ujala-16 seed treatment with ZnO NPs at 120 ppm (4.68 µmoles g−1 FW) grown under 50% FC.
Moreover, the activity of antioxidants such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and ascorbate peroxidase (APX) are increased in plants when grown under DS conditions, while ZnO NPs also increase the antioxidant contents in wheat. However, maximum CAT (446.68 Units m
−1 g
−1 FW), SOD (617.08 units m
−1 g
−1 FW), APX (2.68 ABA digested g
−1 FW h
−1) and GPx (165.66 units m
−1 g
−1 FW) activity is recorded for untreated Ujala-16 under DS conditions that are statistically on par with Zincol-16 under the same set of experimental conditions. Seed treatment with ZnO NPs at 40 ppm shows higher antioxidant activity as compared with the higher doses of ZnO NPs (
Table 4).
Similarly, DS also affects the nutrient concentration in wheat plants of both cultivars as N, P, K, Ca, Mg, Zn and Fe in plants are significantly reduced (
Table 5 and
Table 6). However, SP with ZnO NPs remain effective in enhancing the uptake of N, K and Zn while having a lesser impact on P, Ca, Mg and Fe. The maximum concentration of P (5.82 g kg
−1 FW), K (16.31 g kg
−1 FW), N (7.50 g kg
−1 FW) Ca (19.39 mg kg
−1 FW), Mg (2.80 mg kg
−1 FW), Zn (25.32 mg kg
−1 FW) and Fe (0.15 mg kg
−1 FW) are recorded for Zincol-16 under well-watered conditions (100% FC) and SP with 120 ppm ZnO NPs, followed by Zincol-16 and Ujala-16 when seeds are primed with ZnO NPs at 160 and 120 ppm, respectively. In wheat seeds treated with a high concentration of ZnO NPs (160 ppm) the concentration of P, K, Ca, Mg, Zn and Fe decrease when compared with 120 ppm of ZnO NPs. Moreover, results reveal that Zincol-16 is found to be more responsive to SP of ZnO as compared with Ujala-16.
4. Discussion
The results of this research trial remain in line with the postulated hypothesis that drought mitigation through SP of ZnO NPs might be developed as a biologically viable strategy to boost wheat growth and its physiological as well as antioxidant mechanisms. The results show that drought stress (DS) decreases agronomic traits while seed priming (SP) with ZnO NPs enhances wheat growth and development under well-watered conditions (100% FC) and DS (50% FC). The significant change in shoot and root lengths, their fresh and dry weights, and leaf area per plants in response to SP with ZnO NPs can be attributed to their physiological and biochemical roles during germination and vegetative growth of wheat [
6]. Similar to our findings, it was inferred that ZnO NPs improve the vegetative growth of crop plants depending on the NP’s concentrations, however, the underlying mechanisms still await further in-depth research [
26,
43]. Likewise, it has been inferred that ZnO NPs, when applied in optimum doses, could be more effective in biosynthesizing various endogenous hormones, which tend to mitigate the adverse effects of DS [
21]. Additionally, it has been opined that SP with NPs initiates the biosynthesis of hormones such as gibberellin and auxins, which triggers the root and shoot lengths along with fresh and dry weights of crop plants by off-setting the deleterious effects of water scant conditions [
43,
44]. Moreover, similar to our findings, SP with ZnO NPs was reported to have synchronized the germination and seedling establishment [
26]. It was suggested that the Zn application as the SP agent could improve plant growth in the early stages of development by promoting the biosynthesis of growth hormones.
Besides morphological growth, the result of this research suggest that SP with Zn NPs imparts a significant impact on plant physiology and growth because the seeds absorbed a greater concentration of NPs [
45]. The increase in plant shoots and root lengths, their fresh weight, and leaf area in plants grown from ZnO NP-primed seeds are presumably increased chlorophyll contents. Improving the biosynthesis process of catalytic and structural components of various proteins, enzymes and co-factor for various developmental pigments was also ascribed to Zn involvement [
46,
47]. Our results are in line with the previously reported findings by Popović et al. [
48] who documented that the application of Zn increases the plant’s fresh and dry weight and height due to an increase in chlorophyll content and nutrient acquisition traits under normal conditions. The untreated seeds gain significantly lower biomass, which might be due to Zn deficiency under normal and DS conditions. Similar findings have also been reported by Ljubičić et al. [
49] whereby low concentration of Zn exhibits a slower rate of growth and reduced seedling vigor compared to chemo-priming with ZnO.
Similar to chlorophyl contents, the DS also negatively affects the leaf water potential and relative water content of wheat cultivars, however, SP with ZnO NPs improves these parameters. This might be due to Zn’s role in boosting different physiological processes (stomatal regulation, photosynthesis, water use efficiency, cell membrane stability and osmolyte accumulation). These findings are significant and are in concurrence with those reported by [
50]. Similarly, it has been inferred that reduction in crop yield is mainly owing to reduced gas exchange rates, uptake of water and leaf water status in plants exposed to DS [
4,
51]. Under DS, our results reveal that relative water content (RWC) are significantly reduced, which is in agreement with those of [
52,
53], who documented that DS decreased the relative water content in maize leaves. However, Zn application increases the RWC significantly. The drastic reduction in leaf water potential might be ascribed to a lower RWC, which might have caused stomata closure [
4,
48,
50]. Additionally, the DS causes a significant reduction in chlorophyll content of wheat plants, which might be due to reduced leaf area, premature leaf senescence, increased leaf temperature and impaired photosynthetic machinery [
54,
55]. However, SP with ZnO NPs increases the chlorophyll content. That increases water uptake and nutrient uptake with the application of ZnO NPs due to better and improved leaf area [
25,
56]. The ZnO NPs might increase the physiological performance and photosynthesis process. In concurrence with these findings, it is revealed that ZnO NPs exhibit a vital role in the biosynthesis of chlorophyll by protecting the sulfhydryl group of the chlorophyll [
57]. The ZnO NPs increase the chlorophyll content by promoting chloroplast development and play a vital role in repairing photosystem by synthesizing a recycling damaged D1 protein [
48,
49]. Overall, the change in the biomass of crop plants are in agreement with the findings of Salam et al. [
46] who state that the NPs ZnO significantly improves the leaf pigments in plants, which enhances the biomass productivity in stressful environment.
Proline content and the activity of antioxidants such as APX, CAT, GPx and SOD, increases in the plants grown under the DS conditions in comparison with well-watered conditions. However, under the well-watered and DS conditions, the increases in proline and antioxidant content are noted for SP with ZnO NPs. This might be attributed to the biosynthesis of Zn finger proteins. ROS scavenging is enhanced by the C
2H
2 Zn finger protein. The C
2H
2 Zn finger protein boosts drought tolerance in plants. Scavenging the ROS is owed to increased activities of SOD and POD in rice by the ZFP245 Zn finger protein. These reduce the deleterious impacts of DS and boost the growth and paddy yield [
58]. Moreover in plants, the DS increases with the C
2H
2 Zn finger protein which imparts drought tolerance in the plant by activating the signaling process and triggering the biosynthesis of ABA hormone [
59]. Therefore, the increase in the expression of Zn finger proteins counters the adverse effects of DS by increasing the synthesis of compatible solutes, scavenging ROS and triggering the signaling pathways. In this trial, the DS decreases the uptake of N, P, K, Ca, Mg, Zn and Fe in plants in comparison with well-watered conditions. Moreover, SP with ZnO NPs remains effective in increasing the uptake of N, K, and Zn as compared with P, Ca, Mg and Fe, which might be due to the negative interaction of Mg, Fe, P and Ca in the Zn absorption on the surface of the root and its translocation from root to shoot in plants [
44,
60].